Performance improvement in pharmaceutical R&D through new outsourcing models
Abstract
The stimulation of innovation in the pharmaceutical industry through outsourcing of research and development (R&D) activities within the drug discovery and development process is analyzed. The empirical data were collected through interviews with experts of pharmaceutical companies and service providers between 2002 and 2005. Additionally, in 2008, the outsourcing behavior of the already interviewed and additional companies was analyzed through desk research. The results show that the outsourcing behavior of traditional and emerging pharmaceutical companies is completely different. Whereas the make-or-buy decisions of traditional companies are mainly competency or know-how-driven, that of emerging companies are primarily capacity or cost-driven. Nevertheless, for both types of companies the cooperation model of “strategic partnership” offers access to high-level expertise while reducing fixed costs and complexity. Within this model, external providers are temporarily integrated into internal R&D teams and thus able to support R&D projects flexibly and more timely.
Introduction
Increasing pressure on the pharmaceutical industry
Global competitiveness is becoming increasingly important for the pharmaceutical industry. Companies are exploring options to enhance the efficiency of the resources they are using at all stages of the whole value chain from discovery research to production and logistics as well as sales and marketing. Especially innovation is recognised as the cornerstone for competitive advantage and is fostered by strong investments in R&D (Achilladelis et al., 2001). Rising costs of pharmaceutical R&D coupled with the increasing pressure of stakeholders demanding steady growth lead to increasing pressure on the output of the innovation pipeline. But drug development and commercialisation is an expensive, lengthy and risky process. Studies published in 2003 report an average pre-tax cost of approximately US$800 million to bring a new drug to the market (DiMasi, 2002; DiMasi et al., 2003). It is estimated that by the time a medicinal product is placed on the market, an average of 12-13 years will have elapsed since the synthesis of the new active substance. Thereby, on average, out of every 10,000 sub-stances synthesised in laboratories, only one or two will successfully pass all the stages to become marketable medicines (EFPIA, 2008).
Performance improvement by outsourcing
The current pressure to increase the output of R&D has created new needs for specialized technologies with the potential to reduce lead times and streamline the drug discovery and development process. Cockburn, Henderson, and Stern (Cockburn et al., 2000) have shown that drug discovery productivity is dependent on the internal organisation of R&D. For these reasons, pharmaceutical companies have been forced to reassess their mode of R&D operation including outsourcing activities (Quinn, 1999; Quinn, 2000). Outsourcing, traditionally thought of as a short-term strategy for demand realization, could be considered to lever the core competencies to increase performance in pharmaceutical R&D. There are good arguments to stress the complementarity between in-house R&D and external know-how (Arora et al., 1990; Arora et al., 1994; Cassiman et al., 2002; Cockburn et al., 1998). For example, Arora and Gambardella examined the complementarity among sourcing strategies of large companies in the biotechnology industry. The access to external know-how may leverage the productivity of internal R&D activities if the organization exhibits a willingness to absorb external ideas (Veugelers, 1997; Veuglers et al., 1999). An important task in innovation management, therefore, is to integrate internal and external knowledge within the innovation process, in order to benefit from the positive effects each activity has on the other. But outsourcing R&D also bears potential risks due to project complexity and loss of flexibility. Studies to clarify the comparative effect of outsourcing in relation to internal improvements within manufacturing processes showed that internal enhancement of manufacturing capability made it much easier to predict improvements in operating performance than outsourcing (Dabhilkar et al., 2008). Generally, outsourcing leads to negative effects when used only as a cost-reducing strategy to improve short-term performance. The consequence may be the loss of internal know-how and expertise as well as higher total costs in the long term. There are numerous examples where insourcing prevented the negative effects caused by bad outsourcing decisions (The Economist, 1996).
Research question and methodology
This paper discusses how the challenges facing the pharmaceutical industry are shaping “make or- buy” strategies within pharmaceutical R&D. Pharmaceutical industry includes also the biotechnology industry which is strongly linked to the pharmaceutical industry. The discussion of outsourcing will use the example of chemical synthesis offered by specialized service providers within the drug discovery and development process. This example is chosen as it covers an important part of the drug discovery and development process and represents the outsourcing mentality in pharmaceuticals very well. The empirical data have been collected through desk research and interviews with managers and experts of 19 different pharmaceutical companies and 12 pharmaceutical service providers in different rounds between 2002 and 2005 (Figure 1). An interview guideline with a reference set of questions was developed to secure the comparability of the answers and to leave enough room for spontaneous answers, which gave a semistructured nature to the interviews. Each interviewee was interviewed in sessions of approximately 60 minutes, whereby most of the interviews were conducted face-to-face and only a few by telephone. Additionally, in early 2008, the outsourcing behaviour in the field of chemical synthesis of the interviewed companies and 61 additional pharmaceutical and biotechnology companies was analyzed through desk research using different public sources (e.g. business databases) and company disclosures (e.g. websites and press releases). Additional telephone calls with persons responsible for chemical synthesis clarified open questions, which could not be answered using other sources.
Results of analysis and interviews
Outsourcing behavior of pharmaceutical companies
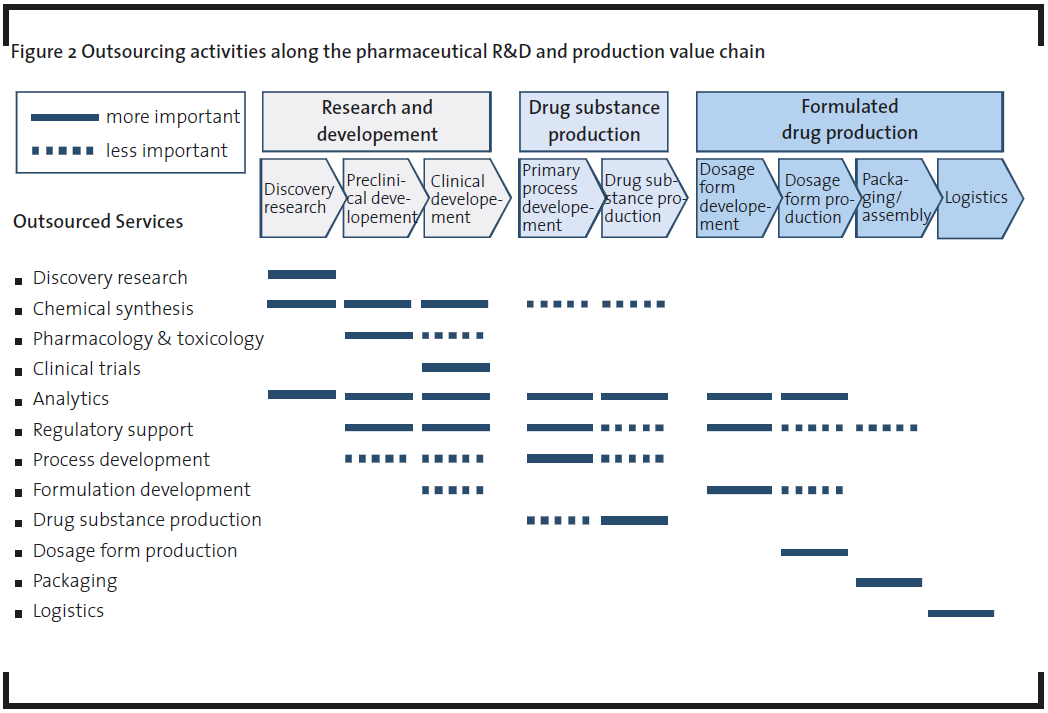
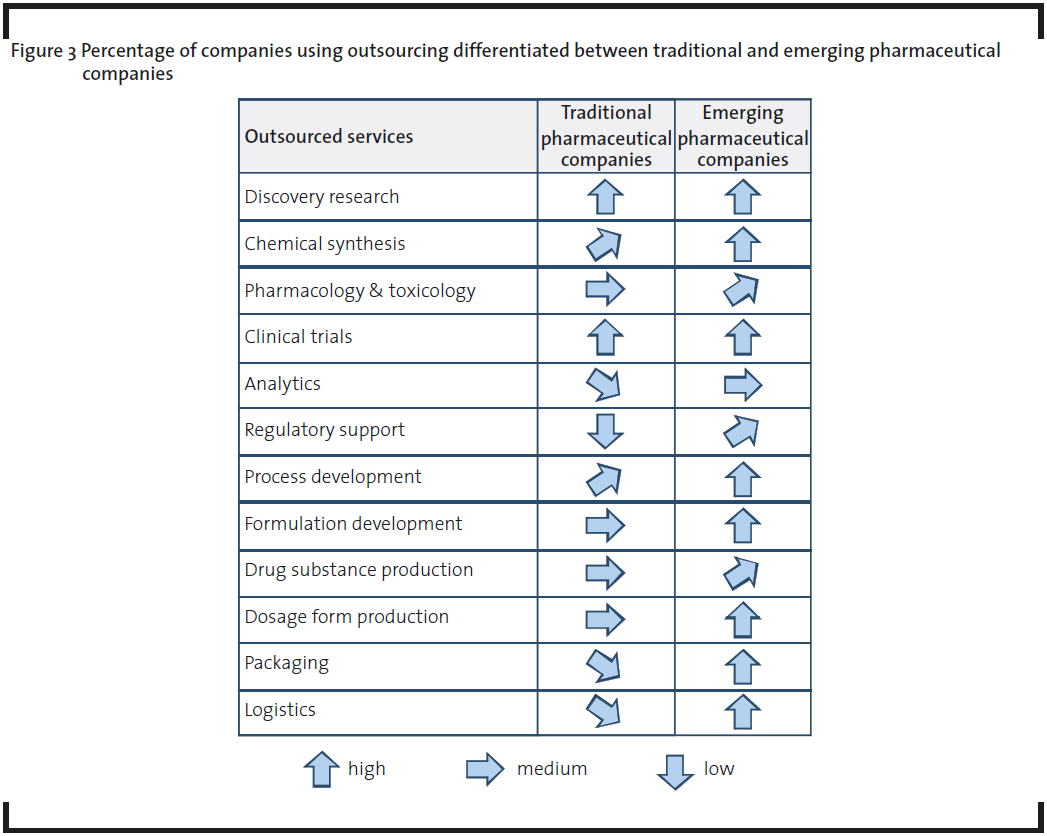
Outsourcing activities are well established along the whole pharmaceutical R&D and production value chain from discovery research to packaging and logistics (Figure 2). Most of the outsourced services are used in one or more process steps of the value chain. The analysis and interviews showed that the differentiation between traditional and emerging pharmaceutical companies is of importance. Traditional pharmaceutical companies, which could be large (“big pharma”) or mid-sized companies, normally cover the whole or most of the pharmaceutical value chain from drug discovery/development up to production and marketing/sales. A widely used term for this kind of company is “Fully Integrated Pharmaceutical Company” (FIPCO). In contrast, emerging pharmaceutical companies are focused on selected stages of the pharmaceutical value chain (Van Arnum, 2008). Most of the biotechnology start-up companies or other technology-driven companies with their roots in R&D are part of this group. Most traditional pharmaceutical companies have their own in-house capacities and the openness for outsourcing is significantly lower compared to emerging companies (Figure 3). They are less interested in buying services due to sufficient in-house capacities. Also cost reduction (reducing fixed costs or reducing people on the payroll) is not so important for the outsourcing of services than always thought. These companies have a high interest in additional, external know-how which is not available in-house or too expensive if it was to be built up internally. Expanding in-house capabilities by external expertise is seen as the most important advantage of using external services. Discovery research and clinical trials are good examples and show the highest outsourcing degree. Within these areas the major requirements in cooperating with services providers are:
- Leading-edge equipment and know-how of the provider while adhering to the highest possible technical standards.
- Clear competence profile of the chemical provider focused on specific segments while being unique and innovative.
- International presence and availability of experts to support the customer worldwide.
- Highly standardized co-operation model covered by general agreements with a precise definition of the ownership of intellectual property.
Compared to traditional companies, the outsourcing level of emerging pharmaceutical companies is generally rather high and in some categories 100% due to low or missing internal resources. These companies see outsourcing as an effective method to capture capacity and expertise without investing much money in in-house resources. In particular, many start-ups lack experience and expertise around drug development, which consequently forces them to rely on external service providers. In doing this, they have the following requirements.
- Lean and flexible development capacities on the side of the providers, easily adaptable to smaller demands.
- Full-service range and know-how around chemical synthesis with capabilities for the support of project management.
- Transparent and flexible cost structures, similar or equivalent to own in-house structures to avoid additional administrative resource burdens.
Cooperation models for outsourced services
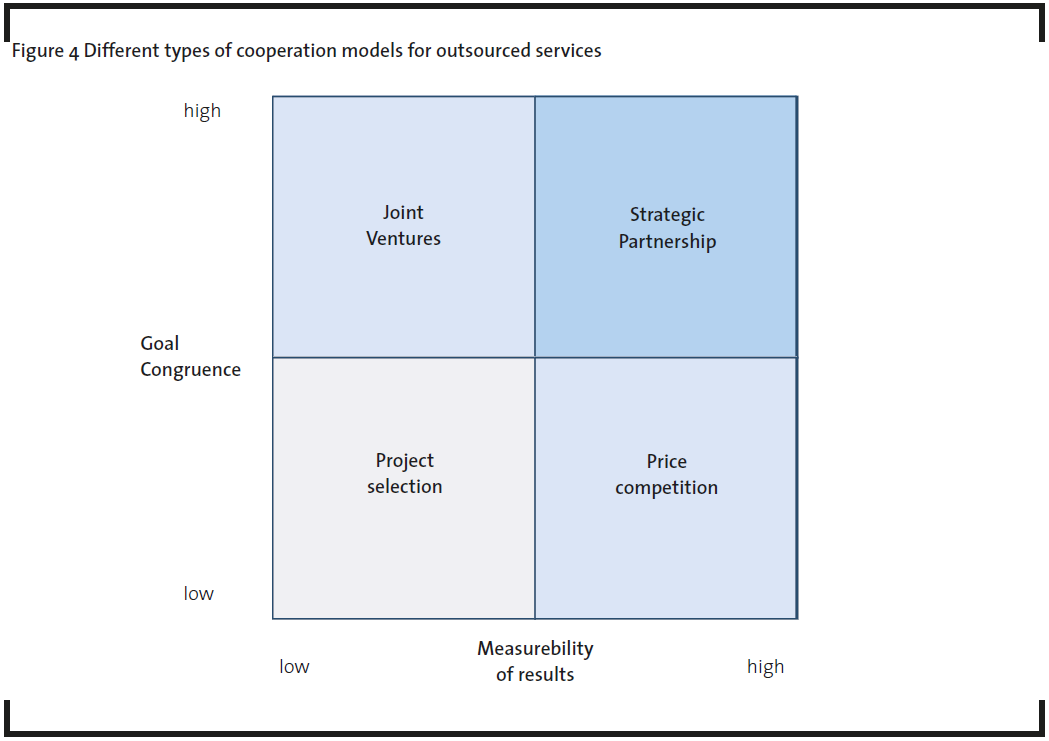
In the areas of pharmaceutical R&D and production, four different co-operation models between pharmaceutical companies as customer and service providers as vendors have been established, depending on goal congruence and measurability of results. There is a simple correlation: the higher the goal congruence, the more trust between the two partners, and the higher the measurability of results, the closer the relationship comes to a traditional customer-supplier relationship (Figure 4).
- Project selection: Selection of service providers on a project-by-project basis from a core list of preselected service providers. The service providers are engaged according to the fit of their core competence to the specific project requirements (e.g. the choice of the best fitting clinical research organisation for the management of clinical trials in a special therapeutic area and/or a special phase of the drug development process).
- Price competition: Long list of service providers systematically put into competition in order to secure the lowest purchasing prices. This model is less strategically oriented, but rather serves to achieve the demand for the most cost-efficient fulfillment (e.g. purchase of standardised analytical services for routine analytical tasks within drug development or quality management). It can be applied successfully only if the outcome can be measured easily.
- Strategic partnership: Strategic links with a handful of preferred service providers who are given preferential “right of first refusal”. A framework contract covers all the relevant services (e.g. contracts with full-service drug discovery service providers like Albany Molecular Research).
- Joint venture: If the results depend on both parties, but contribution cannot be easily attributed, a 50-50 joint venture is a good choice. This approach has not been observed between a pharmaceutical and a service provider. It is a well-known approach in other industries, e.g. in fuel cells, or high-tech in general.
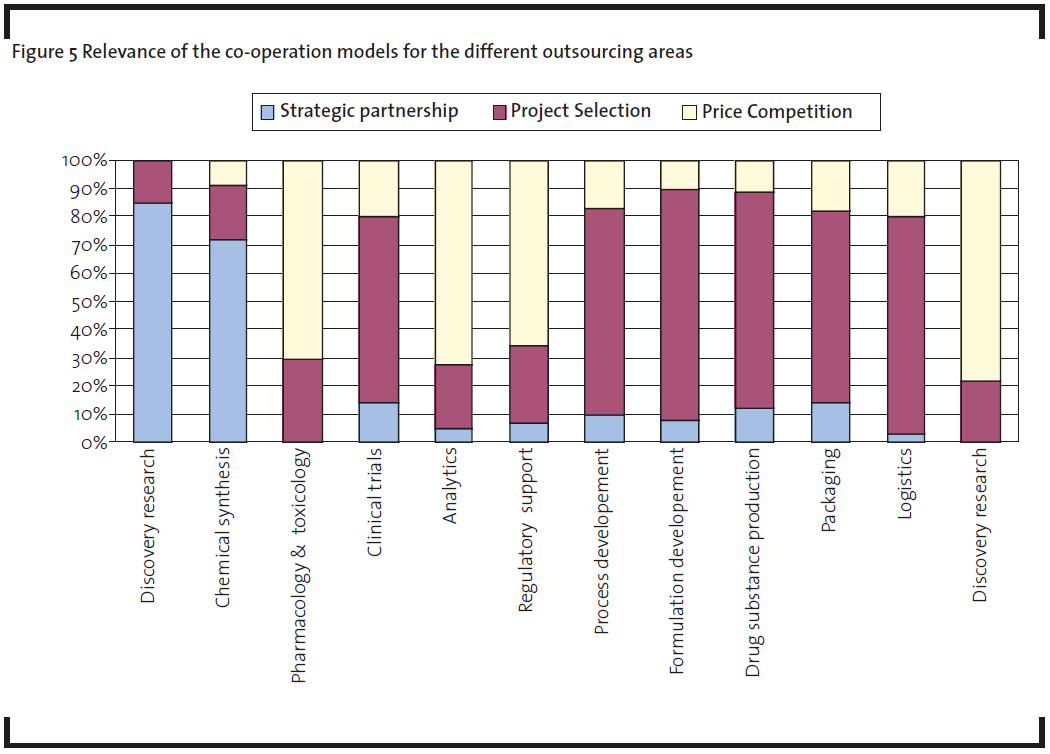
The pharmaceutical industry invests high management capacity in choosing appropriate service providers and committing them to the company to achieve goal congruence. Stringent inspection of the supplier’s facility, quality, best practices, trained staff, and certified processes are crucial in the selection process (Findlay, 2007). Assessment of the service provider’s financial stability is imperative during the selection process. As these suppliers work with various projects from pharmaceutical companies, it becomes crucial to ensure there is no backlog of projects due to financial constraints. Analyzing the relevance of the cooperation models for the different outsourcing areas shows that the most often used cooperation model is “project selection” (Figure 5). The “strategic partnership” model is used mainly in the areas of discovery research and chemical synthesis. ”Price competition” is mainly used for services in the area of pharmacology & toxicology, analytics, regulatory support, and logistics, fields where the deliverables are easy to control. The “Joint venture” model between pharmaceutical companies and service providers is more of theoretical nature, as it is not often found in practice. But there are some joint ventures between service providers, especially to cover emerging markets. A good example is the formation of the joint venture Evotec- RSIL in India between Research Support International (RSIL) and Evotec to design, synthesize and manage compound libraries as a service. The joint venture combines Evotec’s expertise in library design, synthesis, analysis, purification, and project management with RSIL’s synthesis expertise coupled with a low-cost structure in India.
Chemical synthesis services as examples for strategic partnerships
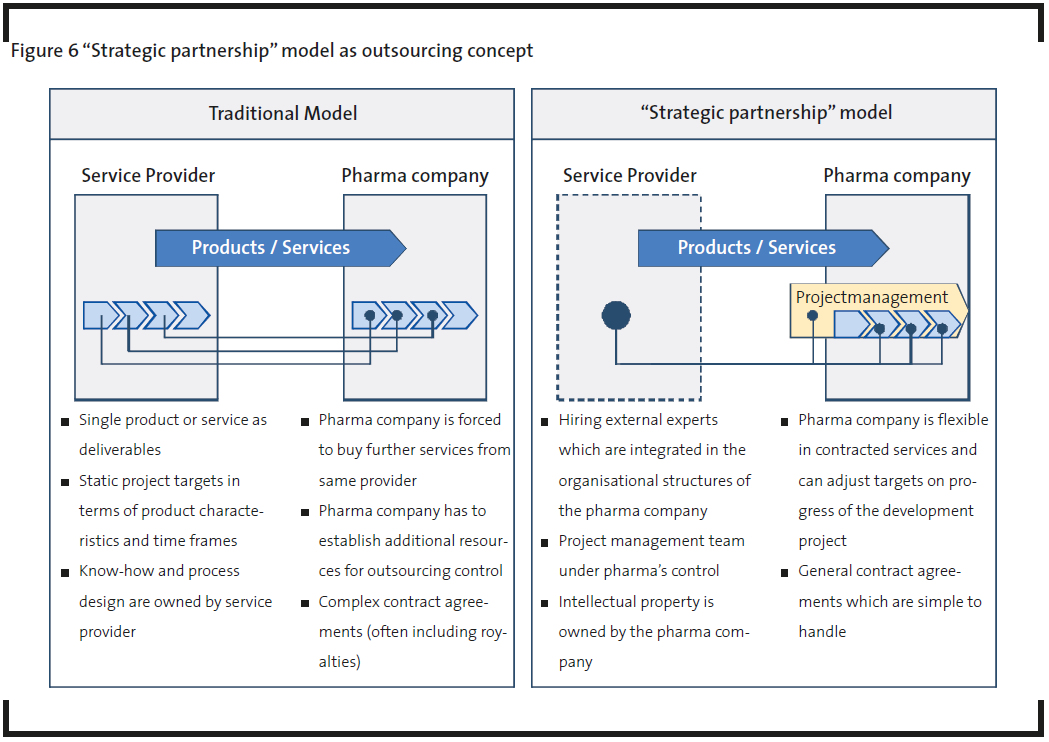
The “strategic partnership” model is analyzed more in detail as it is perceived that this model has the potential to improve the performance of pharmaceutical R&D significantly. For a better understanding of the “strategic partnership” model, the field of chemical synthesis should serve as an example. Traditional outsourcing concepts within chemical synthesis are focused on a single product or service. Only the product (e.g. lead compound or class) is sold exclusively or semi-exclusively to the customer with the provider remaining the owner of the synthesis know-how and process design. Contracts have been rather complex in the past due to opposing views on intellectual property and rigid customer-provider relations. Therefore, both partners are forced to think and act much more result-oriented than react within existing organisational boundaries. Service offerings in outsourcing need to be adapted, while interfaces between customer and service provider, reduced and redefined. A solution is “body leasing”: integrating external experts into internal R&D teams to support R&D projects more flexibly and more timely within pharmaceutical companies. Many “strategic partnership” models are based on service providers hiring out their employees with specialized skills and leaving intellectual property rights in the ownership of their pharmaceutical customers (Figure 6). This represents a switch from isolated service offerings to an integrated platform of support within the customer’s processes and structures. This means that pharmaceutical companies hire in experts for a defined period and integrate them into their in-house R&D structure. Hired experts use either their own in-house infrastructure or facilities inside the customer’s organization. A project management team for which the customer is responsible guarantees success of the development project as well as the intensive know-how and expertise transfer. Highly standardized project management is important to ensure success. This addresses the pharmaceutical industry concerns of minimizing third-party activities for critical path activities through highly standardised processes.
Experiences and learning effects
Cost, time, and innovation are the levers to improve R&D performance and R&D outsourcing could give the mentioned levers a positive impact. The positive effects of outsourcing are enhanced if the supplier is used to supplement existing core competencies (i.e. to free resources in order to invest in higher internal capability). Besides limiting fixed costs, service providers can often provide the expertise and know-how in a more flexible and cost-effective way than internal resources. Furthermore, the complementarity between in-house R&D and external know-how creates additional benefits regarding the quality of research and services. Therefore, the right strategic partner could not only offer cost advantages, but also quality improvement and innovation, and the “strategic partnership” model guarantees a high internal competence level in the long term.
But nevertheless, some aspects like perceived (or real) difficulties to transfer know-how and issues with intellectual property situation are seen as major obstacles for outsourcing. Service providers should react to the concerns of pharmaceutical customers with a best practice approach which includes the following aspects.
- Complexity and efficiency: definition of highly standardized and transparent processes and contracts.
- Co-operation and communication: project management in close vicinity to the pharmaceutical company and not only offshore lab resources (e.g. in China or India).
- Costs and invoicing: establishing full cost transparency and an easy invoicing process.
- Flexibility and quality: high flexibility regarding project execution with stringent quality control.
- Exclusivity and secrecy: clear and transparent rules regarding the engagement in projects of direct competitors.
- Intellectual property: cooperation agreement leaving all critical IP at the pharmaceutical company.
If these aspects are handled properly, the professional market for highly specialized services and the flexible structures within the services networks make pharmaceutical research more efficient. In the future, highly specialised research service providers will play a more important role and integrative part of the processes in the pharma industry. The result is that there has been an increase in drugs introduced to the market over the past years after the number reached a low point shortly after the turn of the Millenium.
References
Achilladelis, B., Antonakis, N. (2001): The dynamics of technological innovation: the case of the pharmaceutical industry, Research Policy, 30(4), p. 535-588.
Arora, A., Gambardella, A. (1990): Complementarity and external linkages: the strategies of the large companies in biotechnology, Journal of Industrial Economics, 38(4), p. 361-379.
Arora, A., Gambardella, A. (1994): Evaluating technological information and utilizing it: Scientific knowledge, technological capability and external linkages in biotechnology, Journal of Economic Behavior and Organisation, 24(1), p. 91-114.
Cassiman, B., Veugelers, R. (2002): Complementarity in the Innovation Strategy: Internal R&D, External Technology Acquisition, and Cooperation in R&D, IESE Business School Working Paper, no. 457.
Cockburn, I. & Henderson, R. (1998): Absorptive capacity, coauthoring behavior and the organisation of research in drug discovery, Journal of Industrial Economics, 46(2), p. 157-182.
Cockburn, I., Henderson, R., Stern, S. (2000): Untangling the Origins of Competitive Advantage, Strategic Management Journal, 21(11/12), p. 1123-1145.
Dabhilkar, M., Bengtsson, L. (2008): Invest or divest? On the relative improvement potential in outsourcing manufacturing, Production Planning & Control, 19(3), p. 212- 228.
DiMasi, J. (2002): The value of improving the productivity of the drug development process: faster times and better decisions, Pharmacoeconomics, 20(3), p. 1-10.
DiMasi, J., Hansen, R., Grabowski H. (2003): The price of innovation: new estimates of drug development costs, J. Health. Econ., 22(2), p. 151-85.
European Federation of Pharmaceutical Industries and Associations (EFPIA) (2008): The Pharmaceutical Industry in Figures, 2008 Edition, Brussels.
Findlay, S.M. (2007): Outsourcing in pharma, Pharmaceutical Technology Europe, 19(5), p. 13-14.
Quinn, J.B. (1999): Strategic Outsourcing: Leveraging Knowledge Capabilities, Sloan Management Review, 40(4), p. 9-21.
Quinn, J.B. (2000): Outsourcing Innovation: The New Engine of Growth, Sloan Management Review, 41(4), p. 13-28.
The Economist, December 21st (1996): p. 113-115.
Van Arnum, P. (2008): Outsourcing Strategies of Emerging Pharma, Pharmaceutical Technology, 32(10), p. 48-53.
Veugelers, R. (1997): Internal R&D expenditures and External Technology Sourcing, Research Policy, 26(3), p. 303-316.
Veugelers, R. & Cassiman, B. (1999): Make and Buy in Innovation Strategies: Evidence from Belgian Manufacturing Firms, Research Policy, 28(1), p. 63-80.