New biocide active substances: needs and challenges in the EU as viewed by industry
Abstract
Emerging regulatory initiatives in the EU are driving towards more environmentally safe chemicals, used as such or in a wide range of products and applications. The aim of the regulations is also to foster and support the emergence of new or safer alternatives and to drive innovations thereof. Biocides are chemicals,which are used in a vast and steadily growing number of applications in order to preserve product safety and quality, however, the number of the Active Substances (AS) used in biocides is decreasing in the EU concurrent with the implementation of the Biocidal ProductDirective (BPD). Accordingly, the present study attempts to elucidate views of representatives of the biocide industry in order to identify some of the present drivers and challenges of new AS development in the different biocide application areas, with emphasis on the economic feasibility of safer biocide development in the future. Notably, the costs of vertebrate testing are amajor factor in development of new AS. Therefore, an evaluation of the costs of such tests and their total proportion of total AS development costs is also discussed. Industry expectations for the implementation of the BPD and impacts thereof are presented.
Introduction
Biocidal products are Active Substances (AS) and preparations containing one or more AS, put up in the form in which they are supplied to the user, intended to destroy, deter, render harmless, prevent the action of, or otherwise exert a controlling effect on any harmful organism by chemical or biological means (EC, 1998). Biocides are used in a vast and steadily growing number of applications from foodstuffs to paints and marine construction (e.g.Waterman et al., 2005; Raczek, 2005). However, despite the growing number of applications the number of available biocide chemistries i.e. Active Substances (AS) is decreasing in the European Union (EU) concurrent with the implementation of the Biocidal Product Directive (BPD) 98/8/EC (EC, 1998; EC, 2003). Moreover, other restrictions due to the acknowledged toxicity and/or eco-toxicity of AS such as Tri Butyl Tin (TBT) in antifouling products or Copper Chromium Arsenic (CCA) for wood preservation have created voids on the biocide market (Bruns et al., 2005). Currently, safer replacements for the out-phased AS are screened for from the existing selection of AS or from the use and development of alternative non-biocidal approaches. Thus, on one hand demands for eco-efficiency may render product raw materials more susceptible to biodeterioration, whereas on the other hand, there is increased concern of development of resistance of target organisms to the existing selection of AS (Maillard, 2002). Accordingly, current trends pose significant challenges to the development of new AS, where profitability and costs due to EU regulation is also a major issue (Bruns et al., 2005).
Current EU regulatory initiatives aim at driving the chemical industry towards more environmentally friendly, sustainable, and safe products and processes as well to concurrently foster innovations within the EU markets (EC, 2006; EC, 1998). The role of regulations as drivers of innovations in the chemical industry is evident (Frohwein and Hansjürgens, 2005), and e.g. initiatives such as the Montreal protocol have boosted the development of more environmentally safe product alternatives (Bonnet and Lacroix, 2006). Moreover, the presently implemented EU directive 2004/ 42/ EC on paints, sets ambitious targets for striving towards the development of low Volatile Organic Compound (VOC) paints (Mast et al., 2008). On the other hand, in many areas of innovative technologies, regulatory initiatives lag behind technology development (Nieminen et al., 2004). This may lead into situations in which the prevailing technologies are out phased due to regulatory risk reduction although safer replacements are not yet available, e.g. decades after the ban on DDT, no successful replacement for all usages is available to date (Coleman et al., 2008). Such may also be the case with formaldehyde and its derivatives, which are widely used biocides with excellent efficacy properties (Power, 1997), but with risk classification as toxic, potential carcinogen and irritant (EC-JRC, 2009). Hence, it remains to be seen whether they will remain as AS of biocides in the EU market as the implementation of the BPD is completed. Overall, the views and response of the industry to emerging regulations is difficult to anticipate when evaluating the possible benefits be gained by a new regulatory instrument (Pearce and Koundouri,2004). This is clearly a critical issue as development of safer chemistries and substitution with less hazardous alternatives is an industry decision and choice.
The biocide industry, i.e. suppliers of biocides and biocide AS, has stated that the development of new AS is not economically feasible, due to the regulatory demands which pose strict requirements for product safety and efficacy testing (Lindner, 2005; Bruns et al., 2005). Accordingly, the aim of the present paper is to elucidate the most significant obstacles and possibilities for product development of new AS by clarifying whether the industry views AS development as technically feasible, what kinds of AS’s are needed and, more specifically, what are the drivers for new AS development as seen by the industry? Moreover, the study evaluated whether regulatory issues have prevented the development of the desired AS’s and more specifically can define demands of the BPD be identified as obstacles of the development? Furthermore, the cost structure of possible new AS product development was evaluated, for which no previous published data is available. The present study also strives to identify the approximate magnitude of costs that may be viewed as intolerable for new AS development within the EU in accordance with previous statements by the industry (Bruns, 2005), and to present an estimate of the cost level which could be tolerable to the industry.
Theoretical Background
Chemical regulations e.g. REACH aim at reducing the environmental and health risks associated with, or due to, chemicals. Benefits of regulatory actions are primarily aimed at reducing health expenditures caused by chemical exposure (EC, 2006; Pearce and Koundouri, 2004). On the other hand, we argue that, in the case of biocides, reduction of risk of the chemical substance is tied to increasing in risk of product spoilage or biodeterioration by unwanted organisms. Clearly, such results will constitute a financial risk for the industry but may also cause health risk to industry workers or consumers (Ludensky, 2005; Scholtyssek, 2005). These factors emphasize the complex repercussions of reduction of chemical risks.Moreover, the BPD also states “[…] when properly used for the purpose intended, they are sufficiently effective and have no unacceptable effect […] such as resistance development […] no unacceptable effect on the environment and, […] health.”(EC, 1998). Hence, reduction of the chemical risks of biocides may not be acceptable if it results in reduced efficacy towards unwanted organisms.
Consequently, the risks of; 1) over regulation of a substance with minor hazards or 2) under regulation of a notably hazardous substance (Koch and Ashford, 2006) is clearly a very relevant issue with reference to biocide regulation and risk management. Accordingly, we also present that the hazard of a biocide must be evaluated with reference to the balance sought between acceptable of tolerable chemical vs. biological risk. Undoubtedly, the industry will need to approach such an evaluation based on economic sustainability. Moreover, development of resistance of the target organisms is a specific risk which is only associated with the biocidal chemical. Notably, as the target organisms are continuously exposed to the same of similar chemicals at a steady concentration, i.e. chemical risk is constant, the risk of development of resistance to the chemical will increase (Maillard, 2002).
Avoiding or mitigating such an increased risk of resistance development could, however, be avoided by development of new AS. The new AS may even be equivalent to chemical risk of current AS, but would offer an alternative for reducing the biological risk. It is therefore within this context that we will approach the question of enhancing innovation and new product development and current stagnated new AS development. Our theoretical framework, therefore, supports the arguments of Frohwein and Hansjürgens (Frohwein and Hansjürgens, 2005), who demonstrated that the Porter hypothesis for regulation as a driver of innovation and new product development may not be directly apply to the chemical industry.
Methods and Approach
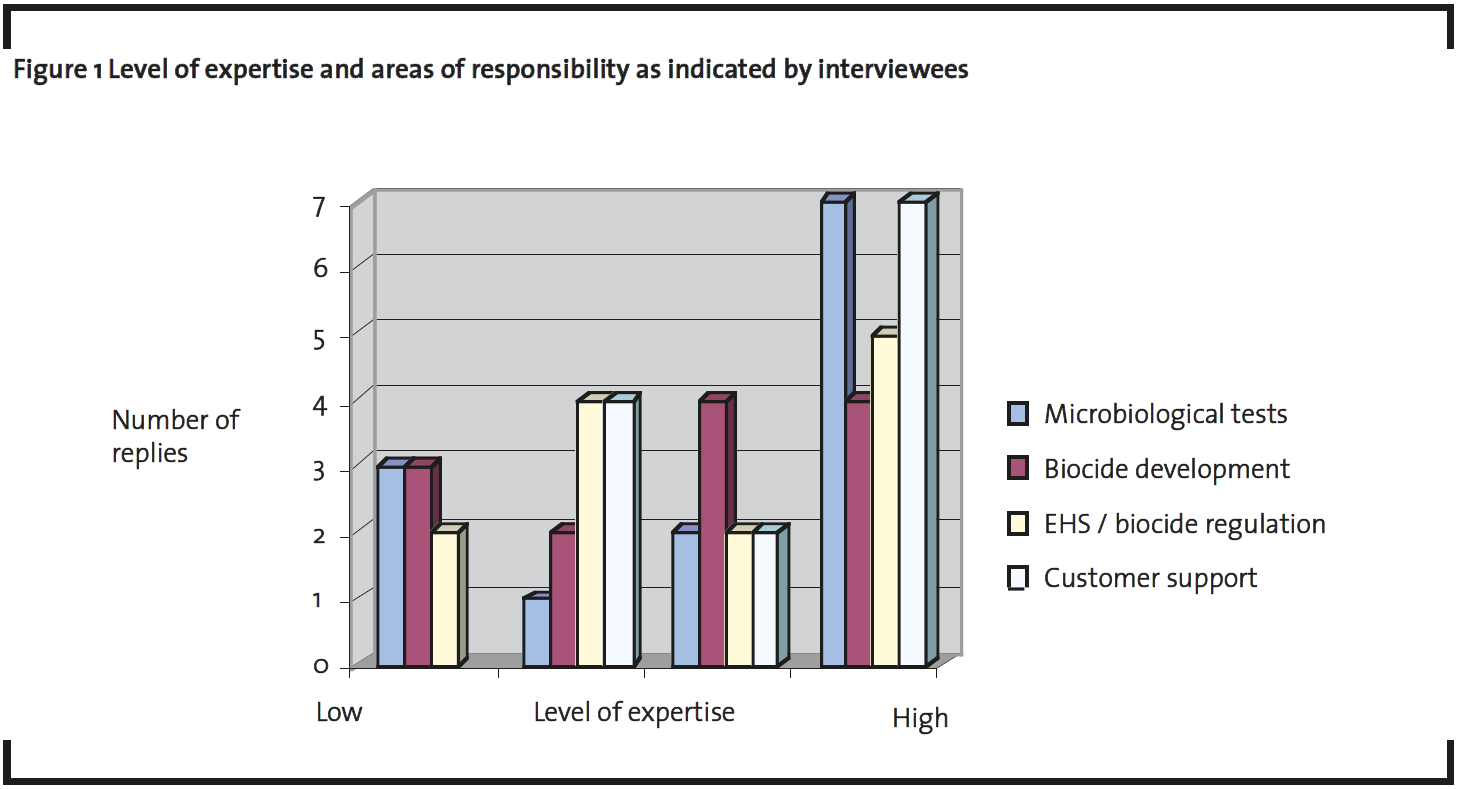
The data was gathered by interviewing 14 representatives of the International Biodeterioration Research Group (IBRG) in 2008. The IBRG is an organization founded under the Organisation of Economic Co-operation and Development (OECD), in 1968 (IBRG, 2009). Its members are representatives of industrial users of biocides, biocide manufacturers, testing laboratories fromboth the private and government sector and academic institutions. A total of ten IBRG experts participated in an oral interview, of which 2 gave answers jointly, giving a total of 9 complete interviews. These ten experts represented companies with a market share of over 50 % of the global biocide market (Anon, 2008a). Moreover a questionnaire was filled in by 4 other IBRG members. These replies to questionnaires were obtained from a global biocide company (1), a small/medium sized European biocide company (1), a European microbiological service provider (1), and a European research institute (1). All interviewees described their roles in the organization by defining how much their work is related to microbiological testing, biocide development, EHS/ biocide regulations and customer support (figure 1.)
The nine biocide supplier interviews were composed of (i) direct questions, (ii) statements and semi-quantitative questions and (iii) a table to fill in during the interview. For analysis of data, the answers to direct questions (n=6) (oral and written) were expressed as numbers of similar answers/total number of answers. With reference to statements such as e.g. “Implementation of BPD will lead to the following”, interviewees were instructed to reply whether they agreed or disagreed by choosing from the following: 0 = not at all; 1 = to minor extent; 2 = to some extent; 3 = completely. Seven statements were presented, ofwhich 3 focused on comparing biocide applications in antifouling, treated wood, process waters, masonry coatings, cosmetics, plant protecting agents, foodstuffs and disinfection (and other areas, if needed). It is evident that some of these application areas are not within the scope of the BPD, i.e. cosmetics, foodstuffs and plant protecting products (EC, 1998). These areas are under different regulations, however, analysis of such regulations were beyond the scope of this paper. Consequently, these areas of application were only included from the point of view of the possibility that a concerted effort towards new AS in these application areas could have significance as a driver for the development of a new AS also in applications within the scope of the BPD.
The results were summarized as a) the number of replies for naming each application and b) the value given (0-2). Answers on applications were expressed as a number of replies / total number of answers in all the applications. Finally, the interviewees were also requested to fill in a table of the development costs of a new AS’s and of new biocidal product development. The estimates were given either as direct cost (€ or $) or as proportion of the total cost (%). Cost evaluation for vertebrate testing in accordance with BPD was calculated independently from the interviews. The calculations are based on data obtained from an international testing services company (Anon, 2008b). The lowest cost alternatives for the tests were used for different means of administration (dietary, gavage, dermal). Moreover, the price for one exposure concentration (instead of 3 concentrations) was used for acute inhalation toxicity. Cost of preliminary tests, as well as costs of tests for finding the preliminary dose range, were omitted form the calculations as they are not included in direct test requirements (EC, 1998). Costs for mutagenicity studies were also not included assuming that prior studies in vitro give adequate result. Costs were converted to Euros (1 GBP = 1.253 €, 24.10.2008).
Limitations of the Study
The structure and the approach of the present study set certain limitations. First, the number of interviewees is limited and can not be stated to represent the whole biocide supplier industry. On the other hand, the data collected represents views of major actors of the EU biocide markets and the individuals interviewed had an average of at least 20 years experience in the field in many areas of experience and responsibility (figure 1).
Therefore, the data presented in this study may be viewed as an indication of leading industry perspective with relevance to current and future trends in the EU. Moreover, as these trends are not the topic of scientific publications in general, very little previous data is available for such an industry perspective. Second, an estimation of the economic feasibility of new AS development does not take into account potential revenues. This decision was made, as the aim was to focus specifically on the structure of the development costs and on the share of the costs related to the regulatory requirements, which have been identified as amajor obstacle for new AS development (Bruns, 2005). Moreover, we have addressed the issue of development of new AS profitability (EU) in a previous study (Soirinsuo, 2009). Thus, taking the above limitations into account, the present study offers an industry based perspective on the drivers, challenges and trends of development of new AS in the framework of EU regulations, with specific reference to the implementation of the BPD.
Results and Discussion
Drivers of New AS Development
Fulfilling the technical requirements on new biocide AS is a challenge as they should be harmful to living organisms, but at the same time, be safe for humans and the environment (EC, 1998). However, regardless of this dilemma, all the interviewees agreed either mostly (7/13) or fully (6/13) that it is technically possible to develop new and better AS’s. Regulatory costs were the most notable arguments against new AS development (4/13).
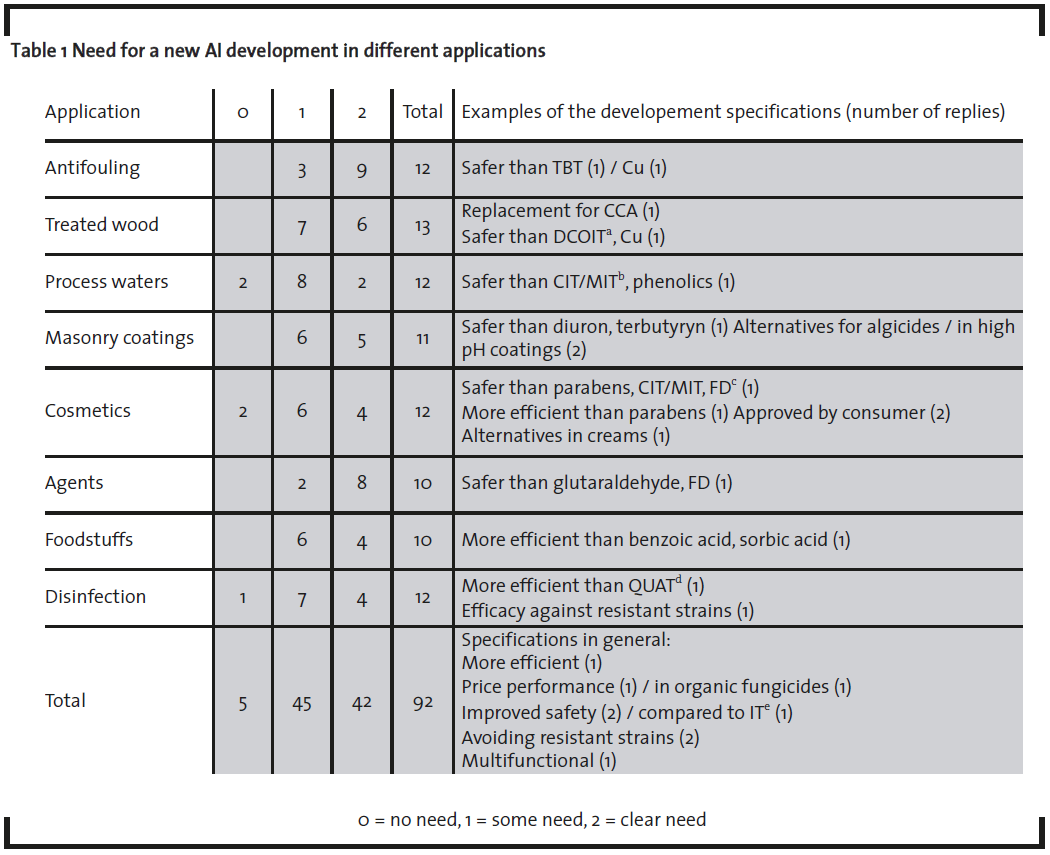
With regard to the need for new AS’s for different applications, the majority stated that there is a need for new AS development (table 1). Only a small proportion answers indicated “no need” (5/92) and the majority indicated some need (45/92) or a clear need (42/92). Moreover, the majority saw a clear need for new AS development in antifouling products (9/12) and in plant protecting agents (8/10). The interviewees were also asked to describe the kinds of specifications that new development should strive for. Based on the answers it became evident that improved safety (without losing efficacy) is clearly the most important driver for the development of new AS’s (11/24). Improvement of efficacy, avoiding the emergence of resistant strains, and widening of the available biocide selection was of equal importance respectively (3/24). A few interviewees also stated that price performance and consumer acceptance are of importance (2 /24 for each). Accordingly, based on the above data, it is evident that development of a new, safer biocide is considered important and also technically feasible. Non-chemical means for control of biodeterioration were not perceived as viable alternatives nor as significant competitors for traditional AS-based chemical biocides.
Regulatory demands and BPD: Present challenges
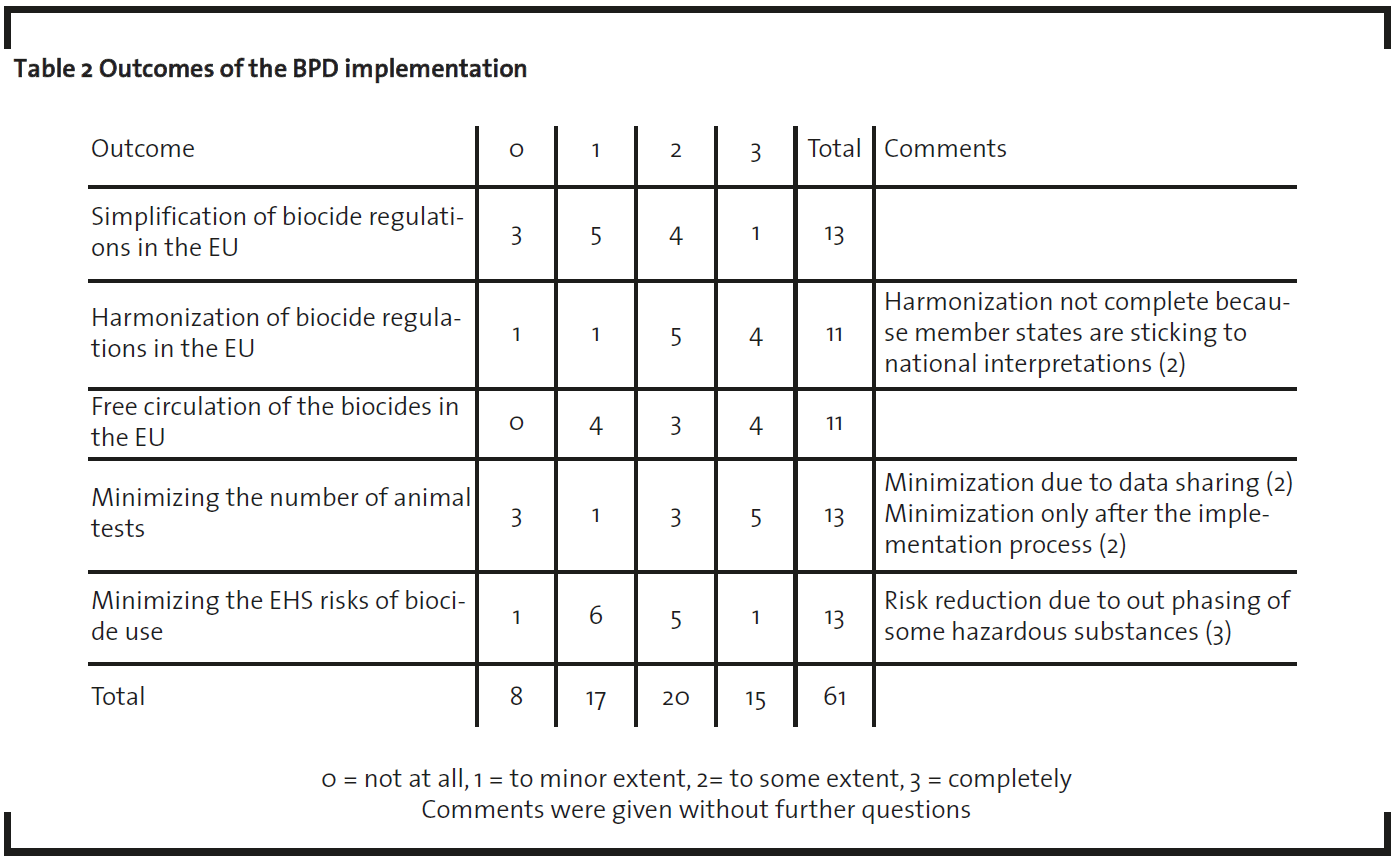
The EU biocide industry is inherently tied to the BPD. A central goal for the BPD is simplification of the national biocide regulations in the EU, none of which covers all the biocide application included in BPD (OECD, 1999; EC, 1998). Other aims include the harmonization of biocide regulations in the EU, enabling free circulation of biocidal products, minimizing vertebrate testing and minimizing the Environment, Health and Safety (EHS) risks of biocide usage (EC, 1998). It is therefore evident that the present status and future expectations related to BPD implementation and impacts thereof play a pivotal role in new AS development. Accordingly, the present study focused on elucidating expectations of the industry as to how well such aims will be accomplished via the implementation of the BPD (table 2).
The minority of interviewees were of the opinion that implementation of the BPD would lead to simplification of biocide regulation (5/13) and only a few regarded harmonization as being completely achievable (4/11) or to take place at least to some extent (5/11). All the interviewees expected that the free circulation of biocidal products would be enhanced at least to some extent after the implementation of BPD. Only some of the interviewees believed that the BPD would lead to a slight reduction in the number of animal tests (3/13), whereas a more significant reduction was predicted by others (5/11) “No reduction at all” replies (3/13) may reflect views on the situation during theongoing implementation of the BPD, whereas other interviewees may have referred more to the expectations after the implementation. Two interviewees stated that number of the animal tests will reduce only after the review process. Half of the general comments on BPD described the BPD as “complicated” or by similar terms (6/12). It was also emphasized that in-house expertise (3/12) and advice from the authorities (5/12) will be imperative for successful management of BPD implementation at company level. On the other hand, a few stated that communication with the authorities is often not successful (2/12) and one interviewee concluded that currently also the authorities are part of the learning process. It is to be expected that as both authorities and industry proceed in this learning process during the implementation of the BPD, understanding of the regulation will improve, and the perceived complexity of regulation may decrease.
Impact of the BPD Implementation on Available AS on the Market
Impact of the implementation of the BPD on the AS selection on the market was evaluated by asking interviewees to name important or interesting AS that are likely to be removed from the EU market due to the implementation of the BPD. Many of the replies to this question (4/7) identified formaldehyde and/or formaldehyde donors (FD). These chemistries are used in numerous applications such as in-can preservation of different products such as polymer dispersions and paints as well as in process fluid preservation e.g. in the pulp and paper industry. “Safer than FD” was also given as one specification for new AS development (table 1). No other out-phased AS chemistries were named as important or interesting by more than one respondent.
Cost Structure of AS and Biocidal Product Development
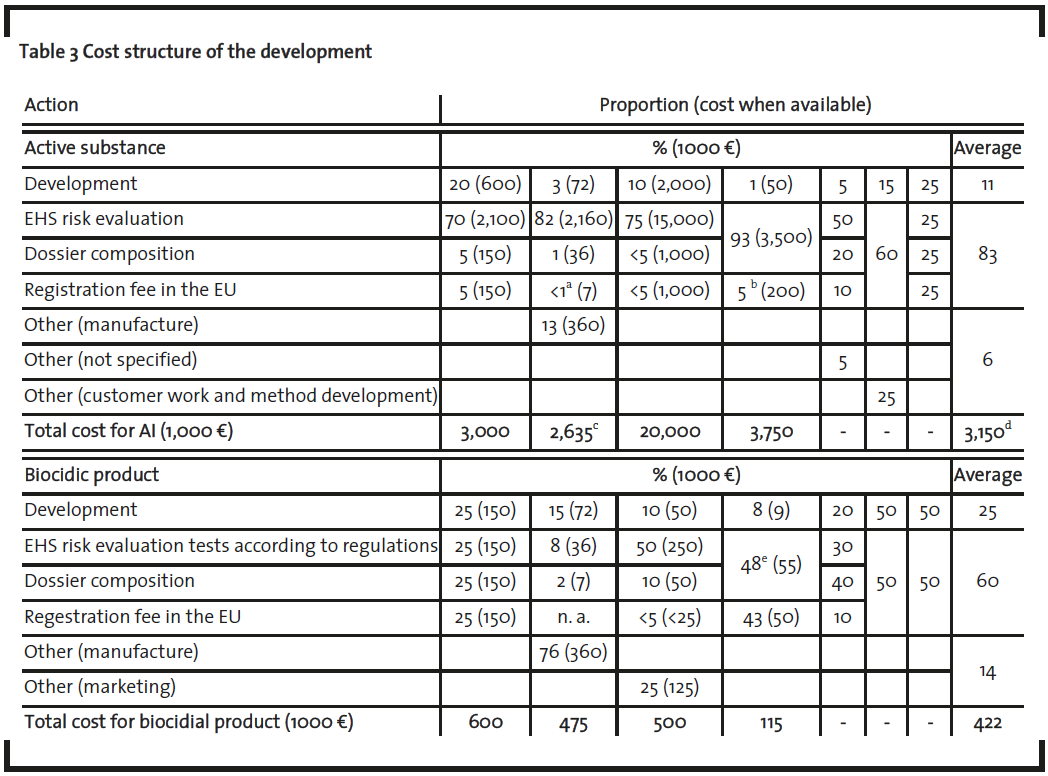
The cost of implementation of regulatory demands has been named as one of the main reasons for stagnant development of new AS (Bruns et al. 2005). Therefore, the aim of this part of the interview was to arrive at a numeric value for what is considered an intolerable cost level for new AS development and what kind of cost estimates would be tolerable. It is to be noted that unlike new AS, new biocidal products are being developed and thus it may be argued that the costs of biocides development are tolerable. The cost structure of the development of a new AS was based on an analysis of data from the oral interviews and the 7 tabular cost estimations given by respondents (table 3). Four of the estimates were given by interviewees as costs (€/$) and three as proportions (%). It is evident (table 3) that the difference between the tolerable cost structure of biocidal products and the intolerable AS development costs is vast (table 3). Interviewees estimated the total cost of a new AS development and a new biocide as being in the range of M€ 2.7- 3.8 (3/4) and M€ 0,1 and 0,6 respectively (4/4). In both cases, the majority of interviewees allocated the main share of the costs to regulatory requirements such as EHS risk evaluation, dossier composition and the registration fee 7/7 for AS and 4/7 for biocidal product development). For new AS development the majority (3/4) estimated the cost as being between M€ 2.2 and 3.5 for EHS risk evaluation including the dossier composition. This falls in the same range as the earlier results obtained by Gartiser et al. (Gartiser et al. 2007). The one exception of the interviewees estimated the cost as being even higher. During the present study, independent of the interviews, we calculated that vertebrate testing would be in the range of M€ 2.4 (Anon, 2008b), for AS testing to fulfill requirements of the BPD. Clearly, these regulatory demands become a major cost factor. Interviewees estimated the costs related to EHS risk evaluation and dossier composition of new biocidal product development as either € 50,000 (2/4) or € 300,000 (2/4). These regulatory issues are also inherently tied to vertebrate testing, with a cost estimate of € 40,000. It may thus be concluded that as the main costs of new AS development are directly linked to EHS risk evaluation testing, a critical challenge is how to reduce these costs without the subsequent increase of the EHS risks which the evaluation specifically strives to control.
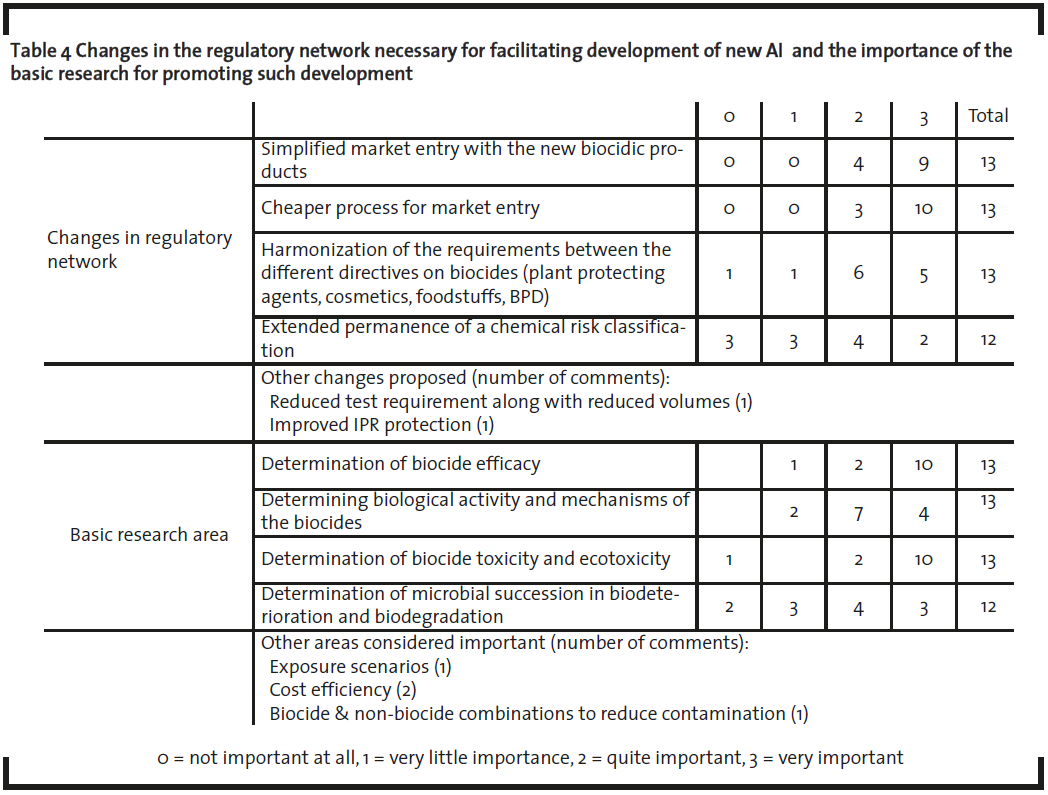
Avoiding Possible Stagnation of AS Development
The interviewees stated that simplification of the regulation is almost as important as cost reduction in enabling new AS development. Harmonization of different regulations or extended permanence of the chemical risk classification (R-phrases, warning labels), were not considered as important. Other regulatory issues that interviewees also stated as supporting interest in new AS development were improved Intellectual Property Rights (IPR) protection (1) and reduction in data requirements in accordance with reduced volumes as implemented under REACH (Regulation (EC)) No 1907/2006 on Registration, Evaluation, Authorisation, and Restriction of Chemicals) (1).
In order to shed light on the more concrete direction on the kinds of regulatory changes that the industry is calling for, the interviewees were asked to name an example of a satisfactory registration procedure of biocides or other chemicals, which also provides adequate safety and environmental information. Interviewees responded by naming the U.S. Environmental Protection Agency (EPA) biocide registration as a sole positive example of such procedures (5/8) as it is considered as being better understood and communicated by both the authorities and the industry (3/8). The other positive aspects of the EPA biocides procedure were better protection of Intellectual Property Rights (IPR) and being based on risk assessment instead of the precautionary principle. This principle comprises a model of anticipatory to protect humans and the environment against uncertain risks of human action (UNESCO, 2005). One comment summarized the EPA procedure as being “more straightforward” as it has been in operation for a longer period of time and thus is better comprehended. Of the respondents who mentioned EPA as the positive example, 3/5 were US-based and the rest (2/5) were based in Europe. Nevertheless, the stagnation of new AS development is also a concern in the US even though the regulatory process may be considered more acceptable.
As the simplification of the regulation was considered a priority for new AS development, the BPD’s objective to simplify EU biocide regulation is well in line with such demands. Presently, however, this has not been accomplished, as the industry considers the BPD complicated. Moreover, the expected future simplification, via learning by experience as the implementation proceeds, maybe seriously hindered by adhering to national interpretations (table 2).
Cost Reduction – Minimizing Toxicity Tests
Reduction of costs was ranked as the most important factor for promoting development of new AS (table 4) where cost reduction is directly influenced by requirements for toxicity testing (table 3). Consequently, basic research in toxicity should be considered equally as important as biocide efficacy itself, which is the most essential and inherent property of biocides. Notably, in the present study, only one interview indicated “no importance at all” for toxicity and eco toxicity research. At the same time this particular respondent ranked exposure scenario research as important, which supports the view on the importance of chemical risks assessment as such.
The clear majority of the interviewees expected a reduction of animal tests to take place after the implementation of the BPD (table 2). Surprisingly perhaps, only one reply suggested similar tonnage trigger structure permitting reduced data requirement with reference to smaller volume as stipulated by REACH (EC, 2006). In conclusion, more targeted toxicity testing research and transparent data on such testing is an important driver for new AS development.
Conclusions
The ultimate goal for new AS development is a “safer than” chemistry of that of an existing AS. The present study indicates that biocide suppliers view the development of such new AS as technologically feasible and there is a definite need for new AS development in many application areas. However, to enable such development simplification of biocide regulation and tolerable development costs are essential. Although simplification of the placement of biocidal products on the EU market is also an important aim of the BPD, industry representatives interviewed in this study were doubtful on accomplishing such a goal. Simplicity of a regulation involves also fluency of communication between the authorities and the industry as exemplified by the functioning of the EPA procedures, with emphasis on direct communication between the parties. In the EU, however, such communication and interaction poses a challenge, as a vast number of national authorities of 27 Members States, are trying to harmonize their work and agree on a common agenda for the interpretation of the regulations (Gartiser, 2007). Consequently, national authorities are largely responsible for the implementation of the regulations and thus play a dominant role in the subsequent communication between all players in the field. According to the present study, it appears that the authorities are considered to be in a learning stage with BPD practices and thus their support for the industry may not be adequate at this time. It remains to be seen what impacts the recent simplification of the registration and centralization of part of the process under the European CHemical Agency (ECHA) will have in the future (COM, 2009).
The costs of toxicity testing on vertebrates according to regulatory requirements were named as the most demanding requirements of new biocides development and basic research in this area was called for. The present study arrived at a cost estimate of 2,400 000 € for vertebrate tests, which represents a major share of the approximately 3,000,000 € of the total cost of development. Although these estimates are only approximations, it is evident that such cost structure together with the ethical considerations gives strong support for the goals of minimizing of vertebrate testing as stipulated by both the BPD and REACH. However a dilemma still exists as, on one hand, both the BPD and REACH consider these tests as the best and most reliable method for evaluating toxicity and eco toxicity, and few alternatives to such testing are currently available. On the other hand, the test requirements for the highest tonnage substances in REACH are reduced compared to the requirements for AS in the BPD. For example REACH defines the chronic toxicity studies on one species as being adequate, while the BPD requires testing in a rodent and a non-rodent species (EC, 1998 and EC, 2006). A step forward has, however, been taken as both BPD and REACH strongly guide data sharing of vertebrate tests in order to avoid multiple testing. According to the interviewees in the present study, such guidance via the BPD were also expected to result in concrete actions of minimizing animal tests. In the case of REACH, the implementation of the obligatory data sharing is verified by centralized control by the European Chemicals Agency (ECHA) of the substances registered. Other data holders than industry may also share the relevant data after the preregistration. Moreover, the possibilities of using alternative test methods or omitting test usage are emphasized in the case of REACH as the Annex XI addresses on the alternatives such as read across of similar substances, Quantitative or Qualitative Structure-Activity Relationship ((Q)SAR) and in vitro testing and recommends their use wherever applicable. Furthermore, complete omission of testing is also acceptable in cases where prior data on human exposure results is available, or the likelihood of only limited exposure can be demonstrated. In the case of BPD similar listing is found in a Technical Guidance Document (ECB, 2000). Finally, in REACH, the reduction of the test requirements accompanied by reduced market volumes of the substances serves the same objective and is equally important in permitting new, small volume chemicals to be brought onto the EU market (EC, 2006).
In conclusion, concise and economically feasible chemical regulation without increasing EHS risks with reference to use of chemicals may not necessarily be a “Mission Impossible”, even though by first glance it certainly appears to be. Active communication between the authorities and industry is a powerful tool for simplifying emerging regulations and their implementation. Decreasing the cost effects of the regulatory demands is a major hurdle, as it calls for development of methods for replacing the most expensive toxicity tests. Thus, research and development of alternative, less expensive test methods is pivotal for the development needs of the chemical industry at large. A number of assumptions have been made in the present study and the trends that we have identified are open for anyone to challenge. As stated also by Pearce and Koundouri (Pearce and Koundouri, 2004) clearly more superior assumptions can be generated, as the methodology, the data, and the limitations of the present study have been made transparent. On the other hand, even as the results of the present study carry merely indicative value, our arguments are very much in line with recent views by Hartung (2009) on the need for development of new testing methods. Hartung (2009) calls for concrete actions in the form of substantial funding for academic and non -governmental organization for the development of such new methods. This underlines the global importance of REACH in exploring an arsenal of tools to reduce the number of vertebrate tests and pushing forth alternative test method development. Consequently, REACH has set the stage for development of tools for the biocide industry for enabling economically sustainable development of safer biocides.
Acknowledgment
The co-operation of the International Biodeterioration Research Group is gratefully acknowledged. The interviewees were able to comment the results prior to submission.
References
Anon. (2008)a: Disinfectant Antimicrobial Chemical Market Share. Freedonia Group.
Anon. (2008)b: Core data requirements as per Technical guidance document of data requirements in support of the EC directive 98/7/EC Annex2A – Active substances- Toxicology tests and Annex 2B – Biocidal Product, Guidance prices by Charles River Inc., October 2008.
Bonnet, P. and Lacroix, E. (2006): The chemistry of fluorinated products at Arkema. Part II from CFCs to HFCs. Actualite Chimique, 301-302 p. 52-55.
Bruns, R., Kaulen, J., Kretschik, M. and Kugler, M. (2005): R&D in material protection: New biocides. Paulus, W., (ed.), Directory of microbicides for the protection of materials, Springer, New York, p. 25-46.
Coleman, M., Casimiro, S., Hemingway, J. and Sharp, B. (2008): Operational impact of DDT reintroduction for malaria control on Anopheles arabiensis in Mozambique. Journal of Medical Entomology, 45(5), p. 885-890.
[COM] Commission of European Community. (2009): Proposal for a regulation of the European parliament and of the council concerning the placing on the market and use of biocidal products, 2009/0076 (cod). Available at http://eurlex.europa.eu/LexUriServ/LexUriServ.douri=COM:2009:0267:FIN:EN:PDF. Accessed 5 March 2010.
[EC] European Community. (1998): Directive 98/8/EC. O J L123, p. 1-63.
[EC] European Community. (2003): Regulation (EC) No 2032/2003. O J L 307, p. 1-96.
[EC] European Community. (2006): Regulation (EC) No 1907/2006. O J L396, p. 1-849.
[ECB] European Chemicals Bureau. (2000): Technical guidance document in support of the directive 98/8/ec concerning the placing of biocidal products on the market, available at http://ecb.jrc.ec.europa.eu/documents /Biocides/ TECHNICAL_NOTES_FOR_GUIDANCE/TNsG_DATA_ REQUIREMENTS/TNsG-Data-Requirements.pdf. Accessed 25 August 2009.
[EC-JRC] European Commission – Joint Research Centre (2009): Details on substances Classified in Annex 1 to directive 67/548/EEC available at http://ecb.jrc.ec.europa.eu/classificationlabelling/search-classlab/. Accessed 24 August 2009.
Frohwein, T. and Hansjürgens, B. (2005): Chemicals regulation and the Porter Hypothesis: a critical review of the new European chemicals regulation. Journal of Business Chemistry, 2(1) p. 19-36.
Gartiser, S., Reuther, R., Reihlen, A., Lüskow, H., Vernon, J. and Zarogiannis, P. (2007): Study on Impact of the implementation of Directive 98/8/EC concerning the placing on the market of biocidal products. Hydrotox GmbH, Freiburg, available at http://circa.europa.eu/Public/irc/env/bio_reports/libraryl=/study_implementation/report_101007pdf/_EN_1.0_&a=d. Accessed 5 December.2007.
Hartung, T. and Rovida, C. (2009): Chemical regulators have overreached. Nature, 460(27), p. 1080-1081.
[IBRG] International Biodeterioration Research Group, (2009) available at http://www.ibrg.org/. Accessed 2 September 2009.
Koch, L. and Ashford, N. (2006): Rethinking the role of information in chemicals policy: implications for TSCA and REACH. Journal of Cleaner Production, 14(1) p. 31-46.
Kähkönen E. and Nordström K. (2008): Toward a Nontoxic Poison: Current Trends in (European Union) Biocides Regulation, Integrated Environmental Assessment and Management, 4(4) p. 471–477.
Lindner, W. (2005): Surface Coatings. Paulus, W., (Ed.), Directory of microbicides for the protection of materials, Springer, New York, p. 347-376.
Ludensky, M. (2005): Microbiological control in cooling water systems. Paulus, W., (Ed.), Directory of microbicides for the protection of materials, Springer, New York, p. 121-140.
Maillard, J.-Y. (2002): Bacterial target sites for biocide action. Journal of Applied Microbiology Symposium Supplement, 92, p. 16-27.
Mast, P., Grisnich, W. and Reuvers, B. (2008): Modified hyperbranched resins: the key technology for low VOC architectural trim paints. Pitture e Vernici, European Coatings, 84(1), p. 13-20.
Nieminen, O., Rahkamo, L., and Nordström, K. (2004): Biotechnology regulation: Views of the authorities, investors and product developers in Finland. Drug Regulation, 38, p. 253-264.
[OECD] Organisation for Economic Co-operation and Development (1999): Series on Pesticides No. 9; Report of the Survey of OECD Member Countries’ Approaches to the
Regulation of Biocides. Environment Directorate, OECD, Paris available at http://www.olis.oecd.org/olis/1999doc.nsf/LinkTo/NT00000B06/$FILE/0E95086.PDF. Accessed 2 September 2009.
Pearce, D., and Koundouri, P. (2004): Regulatory assessment for chemicals: a rapid appraisal cost-benefit approach. Environmental Science & Policy, 7(6) p. 435-449.
Power, E. G. M. (1997): Aldehydes as biocides. Progress in Medicinal Chemistry, 34, p.149-201.
Raczek, N. N. (2005): Food and beverage preservation. Paulus, W., (ed.), Directory of microbicides for the protection of materials, Springer, New York, p. 287-304.
Scholtyssek, R. (2005): Protection of cosmetics and toiletries. Paulus, W., (Ed.), Directory of microbicides for the protection of materials, Springer, New York, p. 263-286.
Soirinsuo,K., Kähkönen, E. and Nordström, K. (2009): Feasibility of Active Ingredient (AI) Development for New Biocides in the EU. Journal of Business Chemistry, 6(3) p.128-136.
[UNESCO] United Nations Educational, Scientific and Cultural Organization. (2005): The Precautionary Principle. UNESCO, Paris, available at http://unesdoc.unesco.org/images/0013/001395/139578e.pdf. Accessed 2 September 2009.
Watermann, B. T., Daehne, B., Sievers, S., Dannenberg, R., Overbeke, J. C., Klijnstra, J. W. and Heemken, O. (2005): Bioassays and selected chemical analysis of biocide -free antifouling coatings. Chemosphere, 60(11), p. 1530-1541.