A Model of Knowledge Sharing in Biomedical Engineering: Challenges and Requirements
Abstract: Technology has always played an important role in medical science by contributing extraordinary advancements to health care. Archaeological excavation shows that the Greek society had already used tools to explore the human body in order to understand human physiology and to diagnose normal and pathologic states. In the last four decades, emerging biomedical engineering sciences have led to the manufacturing of cutting edge medical instruments. Those technical tools are used to enhance clinician’s know-how by providing better knowledge of the human anatomy. A more accurate diagnostic is crucial for medical practitioners in order to suggest an appropriate treatment. For example, the introduction of endoscopes into surgical practice is considered as one of the biggest success stories in the history of medicine.
However, in order to develop suitable medical instruments or procedures, one key issue for successful biomedical research is the ability to understand the requirements as defined by medical doctors. Furthermore, biomedical universities and the biomedical industry, who are the two main actors of the development process of new technologies, need to collaborate and cooperate in an efficient way with medical staff. This ongoing study intends to explore the nature and the role of knowledge transfer between the various stakeholders with the aim to develop innovative medical instruments. Factors inhibiting or facilitating knowledge sharing processes are outlined in this paper.
Introduction
It is well recognised that during the last decade our society has turned to be knowledge oriented (Corso, Martini, Pellegrini, Massa, & Testa, 2006) and that the need to cope with knowledge and its management is one of the main concerns of innovative organizations. Previous studies indicate that there is a link between knowledge management and innovation processes (Arntzen 2006; Brännback, Renko, & Carsrud, 2003; Cormican & O’Sullivan, 2000).
Lately, the emerging concept of a triple helix, representing the way how three institutional spheres (public, private and academic) work together is considered as being the best approach to form an innovation system based on knowledge flow and interactive consultations (Leydesdorff & Meyer, 2000). For example, the European consortium, ArtMed, aims to develop a new portable and real time ultrasound scanning device able to detect early vascular diseases. The partners involved represent three different groups such as universities (Eindhoven University of Technology), hospitals (European Hospital Gorges Pompidou), and manufacturers (Esaote S.p.A). They constitute a typical example of the triple helix concept (Esaote, 2005) in the field of biomedical engineering.
Biomedical engineering (BME) is defined as the application of engineering disciplines and technology to the medical field. It combines engineering expertise with the medical expertise of the physician to help improve patient health care by designing suitable medical devices. As a relatively new discipline, much of the work in biomedical engineering consists of research and development. Therefore, it is crucial that health institutions, research institutes and manufacturers work efficiently together.
One way to ensure success in these types of cross-disciplinary activities is to examine the way scientific knowledge flows between engineers, researchers and physicians while they are involved in an effort to develop or improve diagnostic devices. In this paper, we focus especially on knowledge transfer and sharing processes.
Transfer or sharing of knowledge is no longer considered as a linear process from a source to destination, but it is seen as a spiral pattern of linkages between the three institutional bodies (Leydesdorff, 2003).
Usually universities in the triple helix represent the core partner having the potential to carry out research activities conducting industrial innovation (Grossman, Reid, & Morgan, 2001). However, knowledge and technology transfer from universities to industry is often not optimal. It is acknowledged that it is not unusual to miss opportunities to improve or develop innovative products; this is mainly due to the lack of close and efficient collaboration and cooperation (Brännback, Renko, & Carsrud, 2003; Pérez & Sánchez, 2003).
In addition, researchers at universities who work in an isolated context are often not aware of the needs and challenges of potential target user groups. Thus, some important research efforts can lead either to no concrete outcomes or to results that cannot be exploited or commercialized (Sandelin, 2003).
This statement is even more valid in the biomedical engineering field, where there is a stringent need to ensure close cooperation between University-Hospital-Industry while developing specific tools and procedures to be used by clinicians. The cooperation and collaboration between the three stakeholders involve an effective knowledge transfer and sharing process. Therefore, it is important to determine the factors and channels allowing knowledge and technology transfer to occur (Laestadius, 2004; Leydesdorff & Meyer, 2000; van Baalen, Bloemhof-Ruwaard, & van Heck, 2005).
Obviously, it is important to initially define the nature and topology of the knowledge that is transferred and to be shared before identifying the appropriate channels.
Our research study intends on one hand to explore the nature and the role of knowledge transfer between the various stakeholders and on the other hand to determine the socio-technical factors enhancing knowledge management leading to technology innovation in the biomedical engineering field (Bechina, 2002).
In this paper, we intend to answer the following research question:
- What roles do the use of information communication tools and organizational change play in transferring and sharing knowledge fostering innovative activities in the biomedical engineering field?
The next section introduces the concepts of knowledge and knowledge transfer. Part three describes the context of the study and outlines the challenges and requirements of knowledge transfer within the biomedical engineering field. Finally a model of knowledge transfer and sharing is discussed.
Knowledge and Knowledge Management Concepts
It is usually agreed that no standard definition of knowledge exists. One of the most referenced definitions in the literature is provided by Davenport and Prusak (1998): “Knowledge is a fluid mix of framed experience, values, contextual information, expert insight and grounded intuition that provides an environment and framework for evaluating and incorporating new experiences and information. It originates and is applied in the minds of the knower. In organizations, it often becomes embedded not only in documents or repositories but also in organizational routines, processes, practices, and norms” (T. Davenport & L. Prusak, 1998).
Knowledge is defined as information in a context that is embedded in action (Brooking,1999). It is also seen as a shared collection of principles, facts, skills, and rules (Pemberton & Stonehouse, 1999). In this respect, knowledge is what gives “meaning”, thus the lack of significance leads to disorganized information (Bhatt, 2000). In addition, knowledge is seen as very subjective, because it depends on the beliefs, values, intuition and emotions of the individual (Sunassee & Sewry, 2002).
Furthermore, it is necessary to recognize the different types of knowledge in order to expose its potential contribution to the performance of the organization and to determine the appropriate channels to transfer it (Pemberton & Stonehouse, 2000). Wide-based knowledge definitions highlight the presence of several forms of knowledge; tacit, explicit, implicit and systemic knowledge on the individual, group and organizational levels (T. H. Davenport & L. Prusak, 1998; Dixon, 2002; Inkpen, 1996; Nonaka & Takeuchi, 1995; Polanyi, 1958).
Explicit knowledge has a tangible dimension that can be easily captured, codified and communicated. Explicit knowledge is referred as “know-what”. It can be shared through discussions or by writing it down and stored into repositories, documents, notes and so forth. Instances of explicit knowledge might include a network directory, an instruction manual, or a report of research findings. In contrast, tacit knowledge is linked to personal perspectives, intuition, emotions, beliefs, know-how, experiences and values. It is intangible and not easy to articulate and tends to be shared between people through personal interactions. Tacit knowledge is both social and contextual, therefore it is a complex task to store and communicate it (T. Davenport & L. Prusak, 1998).
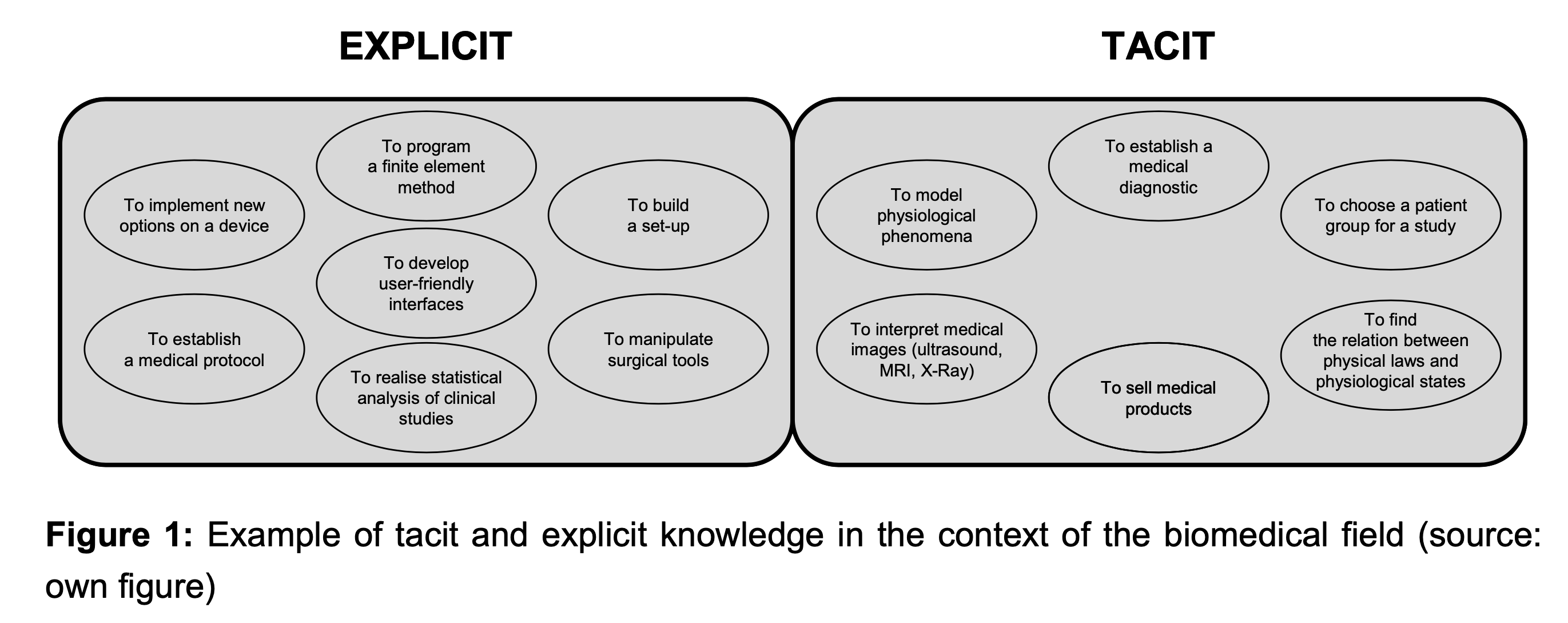
Figure 1 provides examples of tacit and explicit knowledge in the field of biomedical engineering.
For instance, an accurate interpretation of a medical image such as MRI (Magnetic Resonance Imaging) requires tacit knowledge of the physician. This type of knowledge comes from their experience of interpreting and will depend on the contextual setting. Physicians can establish a diagnosis by following a medical protocol that is described usually as a set of rules.
The distinction between tacit and explicit knowledge is important since their management is quite distinctive and requires different channels or means to transfer or to share it.
However, quite often the use of tacit or explicit knowledge is entangled, and it is often hard to have a clear separation between them.
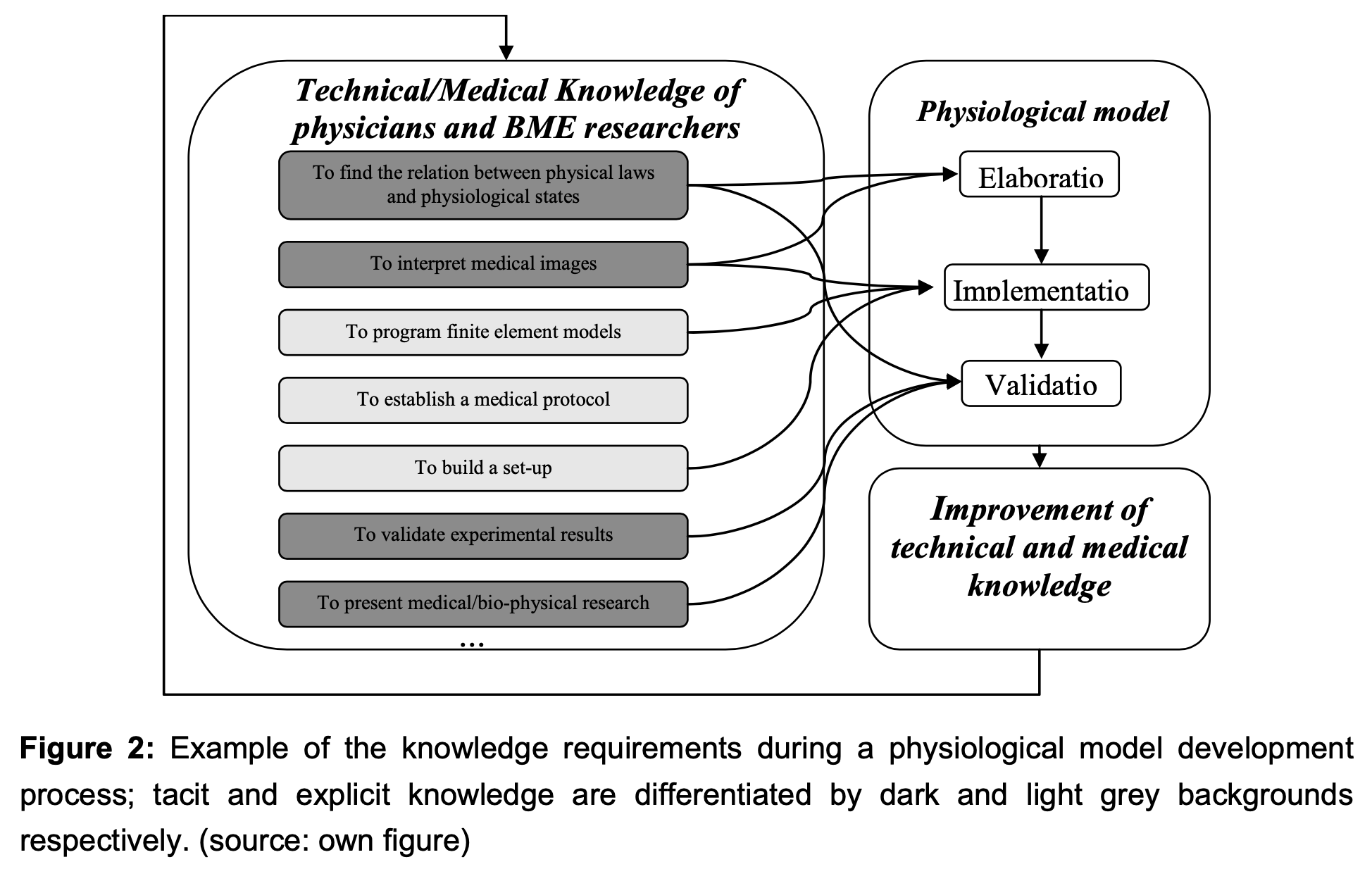
Figure 2 outlines an example of tacit or explicit knowledge needed for the delineation of a physiological model.
In addition, Nonaka & Takuchi (1995) propose a model of knowledge transfer or a creation process (SECI). Figure 3 illustrates four modes of knowledge conversion between tacit knowledge and explicit knowledge. Knowledge conversion starts with the tacit acquisition of knowledge by people who do not have it from people who do. This process is called socialization.
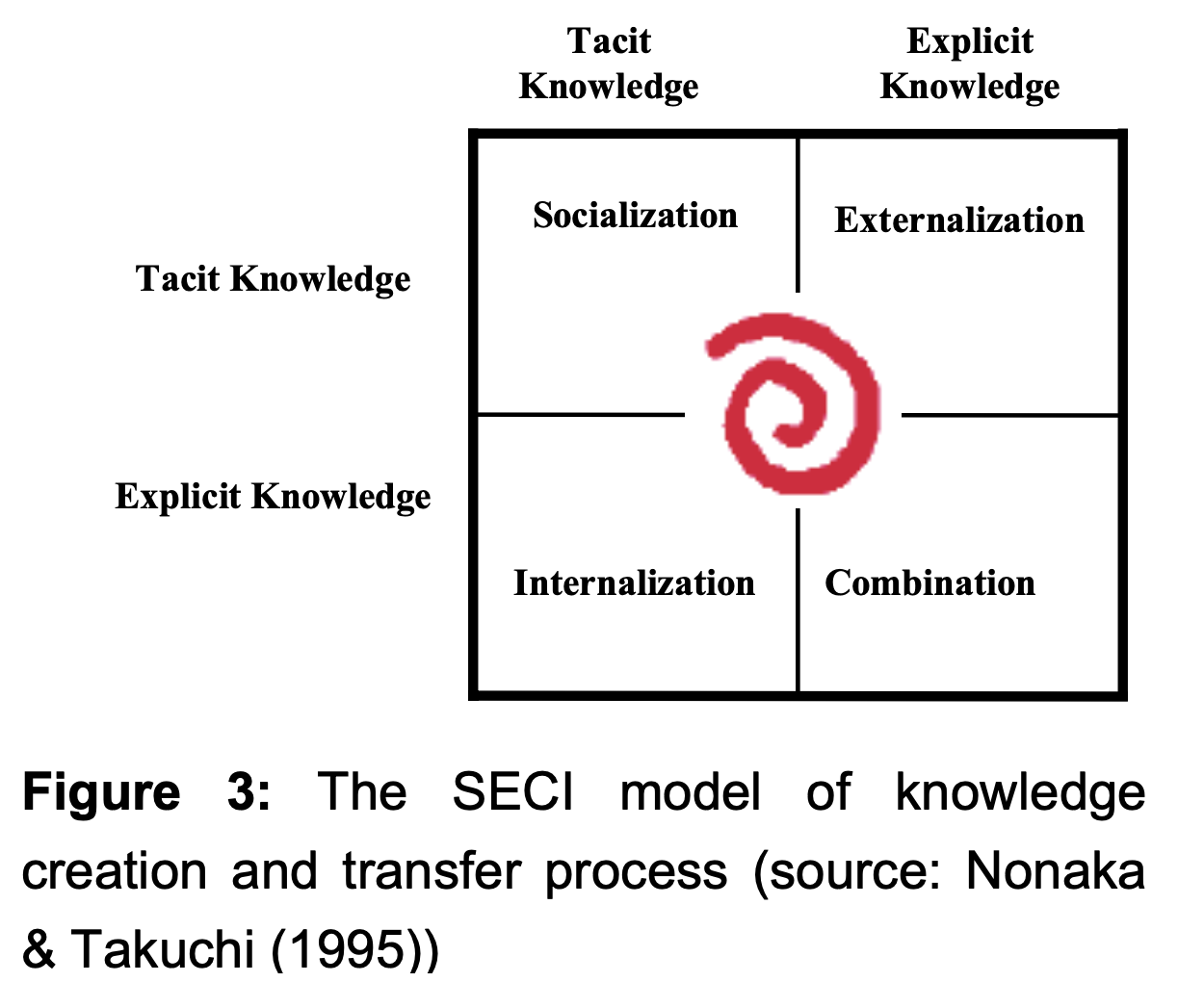
Socialization: from tacit to tacit — It is defined as sharing experiences to create tacit knowledge, such as shared mental models and technical skills. This also includes observation, and practice. It also builds a shared context in which learning and assimilation processes are facilitated.
- Internalization: from explicit to tacit — Explicit knowledge is embodied into tacit knowledge. This is referred to as “learning by doing.” Knowledge is articulated or diagrammed into documents or oral stories.
- Externalization: from tacit to explicit — The process of articulating tacit knowledge into explicit concepts uses metaphors, analogies, concepts, hypothesis, or models.
- Combination: from explicit to explicit — The process of systemizing concepts into a knowledge system triggered by networking
Knowledge management (KM) is seen as an effort to increase useful knowledge within the organization by encouraging communication, offering opportunities to learn, and promoting the sharing and transfer of appropriate knowledge artifacts (McIrnerney, 2002).
“Knowledge management caters to the critical issues of organizational adaptation, survival and competence in face of increasingly discontinuous environmental change. Essentially, it embodies organizational processes that seek to synergistic combination of data and information processing capacity of information technologies, and the creative and innovative capacity of human beings” (Malhotra, 2003).
The high number of different definitions of knowledge management highlights the diversity of the knowledge management processes ranging from knowledge codification, representation, transfer, sharing, classification, search, generation, use and so forth.
In our research study, we focus mainly on the knowledge transfer and sharing process. It is important to understand how the transfer of knowledge from one set of individuals to another is taking place. Alavi and Leidner (2001) emphasize the significance of knowledge transfer by discussing the need for an organization to be successful in its ability to generate new knowledge and to transfer it (Brennenraedts, Bekkers, & Verspagen, 2006).
In the context of high-tech biomedical engineering, we need to comprehend the mechanisms and channels for transferring knowledge in order to enable innovation. A model of knowledge sharing and transfer is discussed in the following section.
A Model of Knowledge Transfer and Sharing in Biomedical Engineering
A Challenges and Requirements
In the context of fast technological changes, successful organisations need to be innovative. The biomedical engineering field deals with cutting edge technology and represents major stakes for society and government. Recent studies demonstrate that medical innovations play a crucial role in improving health and life expectancy. For instance, increases in the life expectancy resulting from a better treatment of cardiovascular disease from 1970 to 1990 have been estimated to bring benefits worth more than $500 billion a year for the United States (Tyler, 2006).
Thus, research and development activities are crucial in the design of innovative medical instruments and will contribute to an improvement of the health care system by enabling a better diagnostic and treatment.
One key issue for successful biomedical research is to ensure an efficient collaboration between the three main actors involved in the development of medical instruments. Figure 4 illustrates the interaction between health care institutions, biomedical engineering (BME) industry and university.
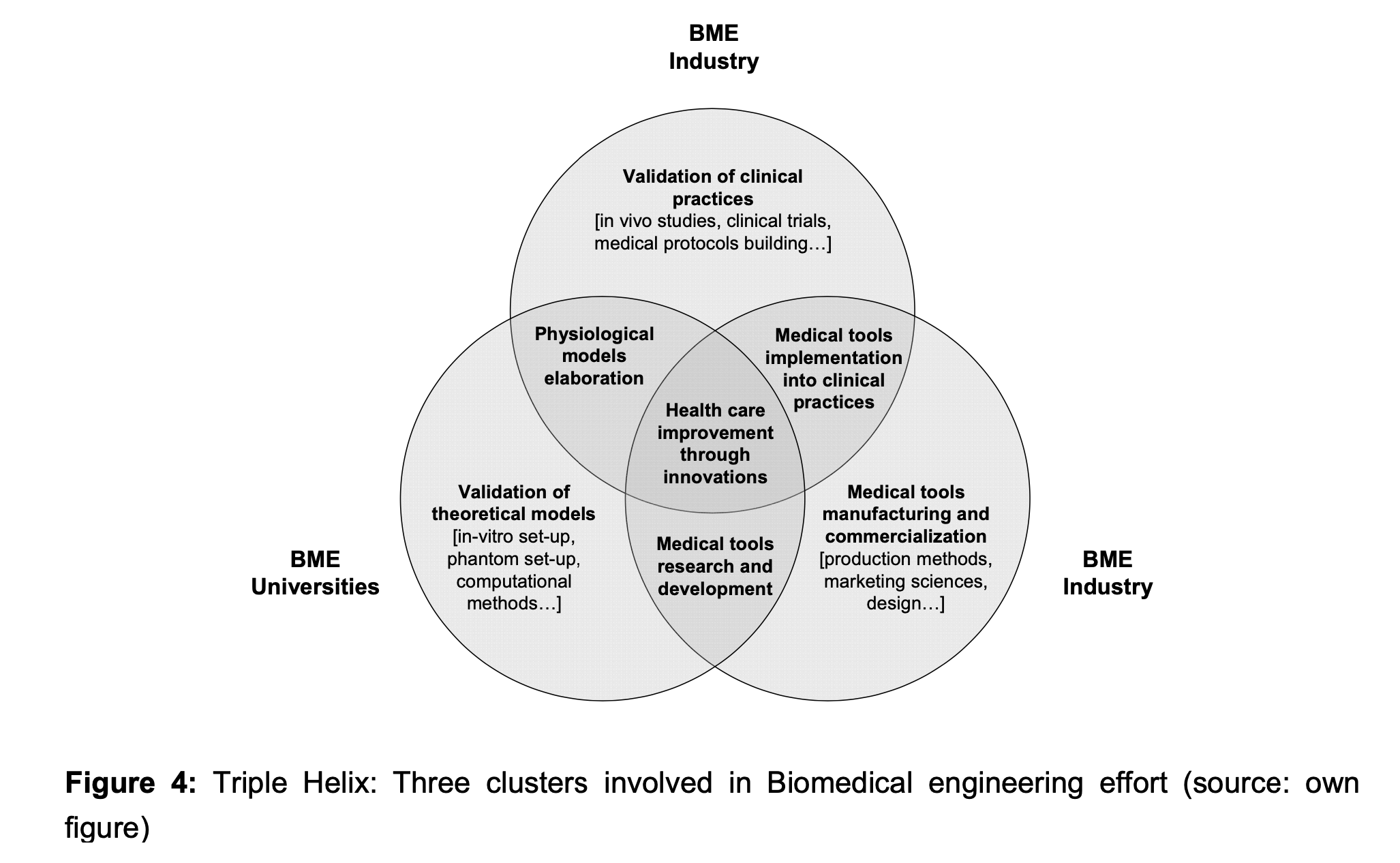
Both biomedical universities and biomedical industries (the two main actors of the production process of new medical tools) should be able to collaborate with medical specialists. Obviously, the main characteristic of a biomedical project is the multidisciplinary context. This, in turn, emphasizes the need to foster a knowledge flow (sharing and transfer) at various levels through stronger and controlled interactions.
These main challenges rely not only on understanding why knowledge sharing and transfer processes are crucial between the different biomedical partners but also on understanding how knowledge sharing is occurring.
BME industry and medical institutions need to collaborate closely while implementing medical instruments into clinical practice. For instance, physicians can observe the physiologic and pathologic states of patients while using medical instruments. Building knowledge is a process that needs to be recorded in order to provide crucial feedback data to companies. This typical phase of knowledge sharing/transfer is important in improving the functionality of medical instruments.
Examples of other collaborative activities involving knowledge flow between medical institutions and BME companies include:
- Requirement of engineering specification
- Validation of new devices by clinical trials
- Training of staff in the usage of medical tools
- Maintenance, quality, and configuration management
- Security, ethical issues, medical legislation
- Quality/price balance
Elaboration of a physiological model requires a strong cooperation between BME universities and medical institutions. The research activities involve both technical and medical knowledge (see Figure 2). For instance, typical knowledge flows will result in the specification of physiological models reflecting the link between medical observations and physical theory. These cross- fertilization activities involve the commitment and understanding from both communities.
The benefits gained from the cooperation between universities and industries are well known. For example, companies are usually profit- driven and the harsh competition might influence their research strategy focus and internal resource allocations. Therefore, one way to acquire crucial knowledge that can build their competence in developing innovative medical instruments is to establish research collaborations with research institutions.
Finally the intersection in the triple helix illustrates that collaborations and an efficient knowledge flow contributes to health care system enhancement. However, observations indicate that although the need to cooperate is well assessed there are still some challenges to overcome and some requirements to fulfil.
For instance, it is vital that technical engineers are able to properly understand the medical context. Usually the first phase in the medical instrument design involves requirements of engineering. This step is the most important since the requirements expressed by medical practitioners should be well understood and analysed in order for the engineers to specify correctly the functionality of the instrument. Therefore, engineers and medical specialists should adopt a common vocabulary that will facilitate communication.
In practice, lack of technical or medical knowledge is the source of misunderstanding or bad interpretations and can induce costly errors while designing a tool.
Traditionally, technical researchers (engineers) were still too little attracted by clinical applications. This was partially due to the low job market offer. However, during the last few years, the increased demand for improved medical devices and systems is said to contribute to the rapid rise in biomedical engineering jobs. Another factor preventing engineers from choosing to specialise in medical tools design is the belief that the strong socio- medical culture will impact negatively on their working process.
On the other side, observations show that health professionals do not use technological systems effectively in their daily routines. In fact, general research studies confirmed that there is rather a latent or open hostility to fully exploit the functionality of information systems or high-tech medical instruments (McDermott & O’Dell, 2001). Furthermore, despite the fact that several ”breakthroughs” in scientific and technological knowledge have been validated through clinical trials, many medical tools are still not adopted by practitioners (Hilton et al, 2002). The reasons usually invoked were related to the instrumental complexity, lack of appropriate training, instruments not really adapted to all patients, high costs, lack of awareness of the potential of some medical instruments, and different medical approaches or protocols adopted by physicians (Le Houx, 2002). These can lead to strong challenges and prevent an effective exploitation of technical knowledge in medical practices.
In addition, end-users complain that medical devices persistently present malfunctions. However, recent studies indicate that the problems were caused rather by medical device usage errors. Indeed, there is widespread evidence that a large number of device usage errors are the result of poorly designed user interfaces (Le Houx, 2002; Todd R. Johnson et al.).
Finally from the business point of view, it is noticeable that academic researchers do not address marketing or commercialization issues and therefore collaborating with industry will ensure that innovative ideas will not be lost. Of course, companies will deal with patent and confidentiality issues and therefore knowledge sharing will not take place spontaneously.
Therefore, collaboration between the three types of organisations is as well characterised by the need to provide a viable business model for the industry. The final purpose for the manufacturer is the production of new medical tools at a larger scale. Of course those business considerations should also be integrated in the set of requirements leading to the technical specification of the medical tools. Other requirements such as ethical issues (compliance) and knowledge of medical legislation have to be considered as well. Collaboration and knowledge flow processes should be clearly outlined for the three partners and some examples of benefits are outlined as follows:
- Quality improvement in development of appropriate medical tools due to feedback from the users (clinicians)
- Development of medical tools that suit the needs of user groups better
- Universities will benefit by testing their concepts and by fundamental research applying their
Industry will benefit from the expertise of top specialist researchers and can expect to improve their own expertise as well as extend their portfolio with new competences acquired while collaboratively designing new medical tools
B Knowledge transfer and sharing model
This research project is still ongoing, but we are already able to draw a general knowledge sharing/transfer model. The delineation of the model is based on:
- Literature reviews
- Informal meetings and discussions with managers, engineers, researchers and physicians
- Punctual observations of work practices and knowledge transfer processes
Document analysis
The model as illustrated (Figure 5) indicates that knowledge transfer and sharing processes can take several forms and occur at different levels in the pyramid.
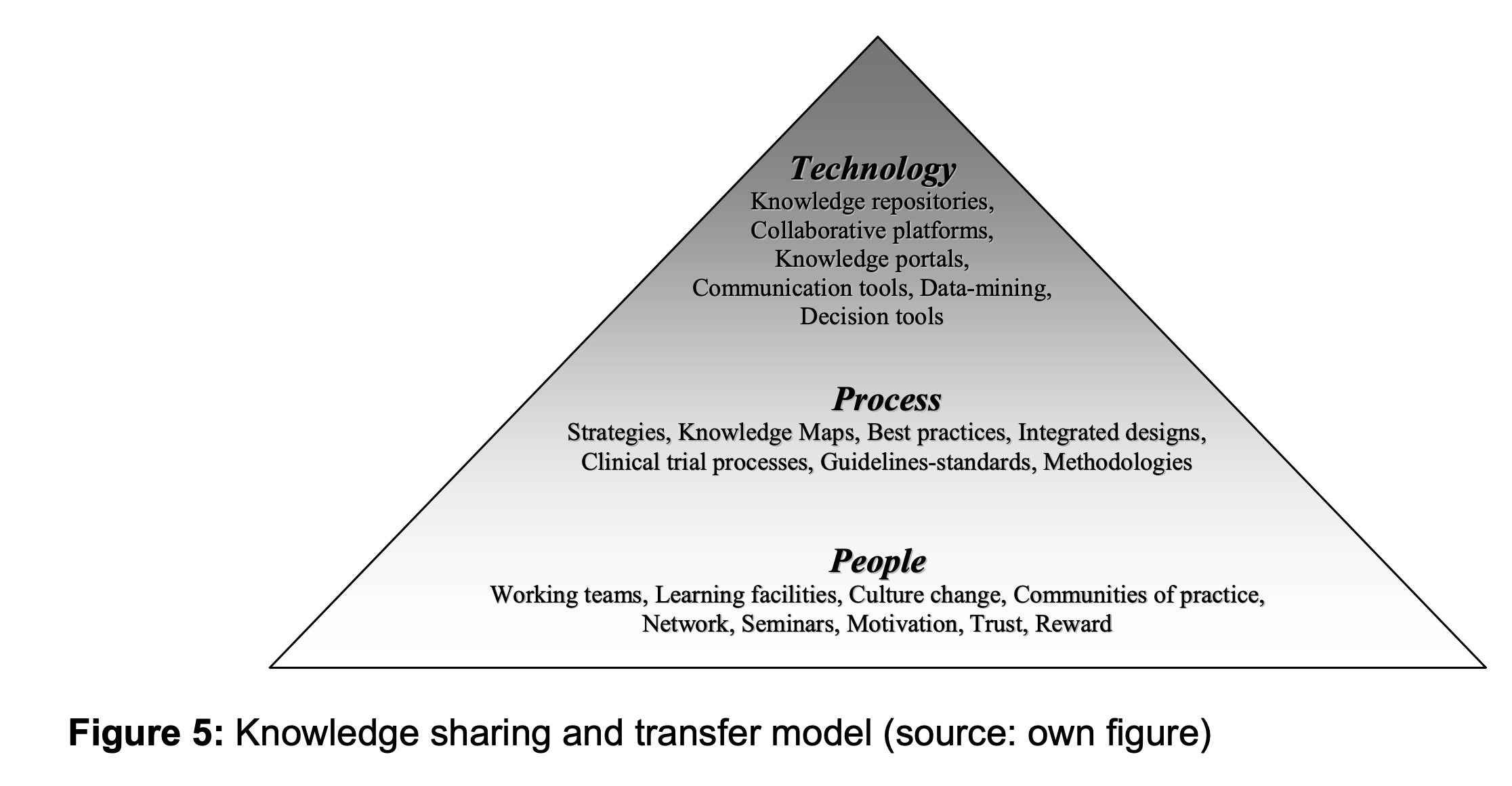
At the bottom level of the pyramid, the people- based layer is the most important and should be the pillar for the knowledge sharing process in a biomedical engineering project. In order to overcome the differences of the three communities, it is crucial to define a framework where technical and medical knowledge can flow without meeting resistance from people. The top level of the pyramid indicates that although technology can be very useful to transfer or share explicit knowledge, the implementation and use of technology should be the last knowledge management focus.
People: Knowledge management is first and foremost an effort to manage, develop, and disseminate knowledge and the full potential of people at an individual, team-based, and organization-wide level. Providing the right cultural environment is the most challenging effort but achievable by enhancing learning facilities, providing a trustful working atmosphere, where collaboration and sharing are encouraged. Other aspects that need to be considered include: motivating and rewarding people that create, share and use knowledge, encouraging communities of practice and promoting network creations.
Processes: Processes play an important role in providing support for any KM implementation. Organisations might need to restructure their internal processes or even the organisation structure itself in order to support KM processes such as knowledge sharing or transfer. Managers must identify knowledge that exists in various forms in the organisation. One way to achieve the goal would be, for example, creating a knowledge map by initially finding out where knowledge resides, point it out and then provide instructions on how to get there.
Technology: Providing a knowledge portal, linking people by e-mail or building knowledge repositories contributes efficiently to sharing knowledge. However, using technology alone will not ensure successful knowledge management as organisational factors such as adequate training needs to be taken into account as well.
Focusing mainly on using technology to support knowledge sharing or transfer, building knowledge repositories might actually slow down the process of sharing. This is mainly due to the fact that many clinicians are reluctant to use information communication tools on a daily basis. Therefore in this specific context, it is important to prioritise the human side by encouraging training of biomedical engineers and clinicians under co-responsibility of educational teams composed of both medical and technical specialists. Such early stage collaborations should foster better communication by exposing involved stakeholders to different cultures. Focus on other processes such as for example, best practices dissemination, needs also to be considered (Bechina , Michon, & Nakata, 2005).
Before implementing some parts of the model, it is crucial to understand and consider the factors that will inhibit the knowledge sharing and transfer. One of our previous studies has resulted in the definition of a generic framework encompassing factors facilitating and inhibiting the knowledge sharing (Arntzen-Bechina 2006; Arntzen-Bechina & Worasinchai, 2006). The next step of the current project is to map early findings within the biomedical engineering field.
However, an analysis of preliminary data collected via informal meetings/discussions and observations outline other factors preventing knowledge flow within hospital or university. They are briefly described below.
Diverse areas of expertise: Although researchers in the biomedical field are supposed to have double competencies and to interface between technical and medical areas, it is still challenging to exchange knowledge. This is partially due to the absorptive capacity of the receiver. Preliminary interviews showed that physicians were not especially interested to know all the technical aspects of a medical device while researchers were not necessarily keen to have an in-depth medical knowledge. This, in turn, increases the problem of communication when they are working together on either user requirements elicitation or on the validation of a medical device. Obviously, the difference in expertise slows down the knowledge sharing/transfer process and is a source of misunderstanding.
Problems with sharing beliefs and cultural norms: Cultural norms differ and represent in fact a major obstacle in knowledge sharing. For example, the dominant cultural factor in a medical system emphasises the individual responsibility of physicians. This creates a climate of non- transparent communication where errors made are not exposed and discussed. It might happen that the border of errors due to either malfunction or bad manipulation is not well clearly defined. Therefore, improvement of a medical device can be hampered. Socio-medical culture deals as well with ethical issues such as specific data of patients that should not be available for a third party. Physician’s value resides in the principle of helping patients and not business corporation, independently of the cost. Therefore, it is recognised that it is impossible to impose business principles or systems thinking on physicians (Richard, 2003).
Another example is related to the clash between academic and business culture. Universities do not emphasise the need for profit or companies cultivate the sense of ownership and patents.
Internal conflicts and difference in interests: Each institution has its own objectives and its internal interest does not necessarily match the objective of a biomedical engineering project. For example, some research institutions aim to advance the fundamental research while health professionals intend to provide the best care for a larger number of patients. Thus, health professional might not be too keen to engage in clinical trials with unpredictable outcomes for the patient’s health. In addition, the companies’ focus is to become a profitable organisation. Therefore, they might have to scale down functionality of medical instruments if the reduction of costs has an impact in terms of selling.
Lack of incentives and rewards for sharing tacit knowledge or using Information Communication Technologies for sharing explicit knowledge
Motivational issues and lack of time: Even if the value of using a medical instrument is well understood by physicians, it is not obvious that they are willing to spend a lot of time on defining users’ requirements or providing systematic feedbacks. The workload in hospitals is considered to be quite heavy and most physicians argue that helping patients is their first priority.
Rigid and or highly hierarchical organizations: Hospitals are usually large and very complex organisations and generate an environment where communication and changes take time and energy. For example, decision making is not a fluid process and it is not unusual that even if it is recognised that collaboration is needed, still there are some barriers to overcome due to many reasons such as regulation on freely accessing data or clinical knowledge.
Conclusion
The paper discussed the need to understand the knowledge sharing process in the biomedical engineering field.
We presented the preliminary results of a research project that aims to analyse the knowledge flow between the three main institutions involved in a biomedical engineering project. The initial data collection is based on literature reviews, observations and informal interviews with key persons from each organisation. This first step has led to the identification of three elements impacting the knowledge sharing process, i.e. people, process, and technology. Some factors facilitating or inhibiting the transfer of knowledge have been outlined and discussed.
We can conclude that a systematic approach is required and should encompass the following steps:
Firstly, it is important to identify the key knowledge workers within the organizations and launch a campaign of communication stressing the importance of sharing knowledge. Some incentives or rewards need to be established in order to motivate all the knowledge workers involved in the process of developing new tools or technology. The third phase should be dedicated to the design of specific sharing mechanisms facilitating the knowledge transfer. One indispensable issue is related to set up some metrics to measure the impacts of knowledge transfer during the design of innovative medical instruments. It is clear, that the choice of metrics relies heavily on the type of initiatives implemented for transferring knowledge. The focus might be either organizational change or the use of appropriate information communication tools.
This study is at an earlier phase, and we intend at a later stage to collect quantitative data from different stakeholders in order to understand the current knowledge transfer that is in place and to provide a set of recommendations in order to improve the flow of knowledge in the triple helix university-hospital-industry
Acknowledgements
The authors sincerely thank Prof. Frans van de Vosse and Dr. Mariëlle Bosboom for their valuable discussions. We would like also to thanks the anonymous reviewers for their valuable comments. We are grateful to Andrew Criswell for his help.
References
[1] Alavi, M., & Leidner, D. E. (2001). Review: Knowledge Management and Knowledge Management Systems: Conceptual Foundations and Research Issues, MIS Quarterly, 25(1), pp.107-136.
[2] Arntzen-Bechina , A., Aurelie. (2006). Yet another Knowledge sharing Framework, International Journal of Knowledge, Culture and Change Management, 5(9).
[3] Arntzen-Bechina, A., Aurelie, & Worasinchai, L. (2006). An Innovative Knowledge Management Approach in Higher Education: A Case Study of Bangkok University, ASIHL-Thailand Journal 9(1).
[4] Arntzen , A. (2006). Knowledge, Learning and Innovation: the quest for a competitive Edge, in: Y. Cader (Ed.), Integrated Knowledge Management: Heidelberg Press Anticipated Publication: September 2006.
[5] Bechina , A., Michon, N., & Nakata, K. (2005, 21-22 November 2005). Pathway to Innovation through Knowledge Management. Paper presented at the ICICKM, the International Conference on Intellectual Capital, Knowledge Management and Organisational Learning, Dubai, United Arab Emirates.
[6] Bechina, A. A. (2002). Une nouvelle platforme dédiée à l ́enseignement à distance, Paper presented at the Journée des réseaux Lyon.
[7] Bhatt, D. (2000). Excellence Model and Knowledge Management Implications [Electronic Version] from http://eknowledgecenter.com/articles/1010/1 010.htm.
[8] Brennenraedts, R., Bekkers, R., & Verspagen, B. (2006). The different channels of university- industry knowledge transfer: Empirical evidence from Biomedical Engineering, Eindhoven: Eindhoven Centre for Innovation Studies, The Netherlands.
[9] Brooking, A. (1999). Corporate Memories, Strategies for Knowledge Management, Thompson Business Press, London.
[10] Brännback, M., Renko, M., & Carsrud, A. (2003). Knowledge intensive innovations management: Networking within and across boudaries, paper presented at the 48th ICSB World Conference, Belfast
[11] Cormican, K., & O’Sullivan, D. (2000, July 17th-19th, 2000). A Collaborative Knowledge Management Tool for Product Innovation, Paper presented at the Managing Innovative Manufacturing Conference, Birmingham, UK.
[12] Corso, M., Martini, A., Pellegrini, L., Massa, S., & Testa, S. (2006). Managing dispersed workers: the new challenge in Knowledge Management, Technovation, 26(5-6), 583-594.
[13] Davenport, T., & Prusak, L. (1998). Working Knowledge: How organizations manage what they know, Havard Business school press
[14] Davenport, T. H., & Prusak, L. (1998). Working Knowledge, Harvard Business School Press
[15] Dixon, N. (2002). The neglected receiver of knowledge sharing, Ivey Business journal, 2004
[16] Esaote, S. p. A. (2005). Art-Med: Arterial medicine: Ultrasound Challenge on Vascular Risk Predictiona nona non–invasive approach invasive approach, from http://www.eureka.be/inaction/AcShowProje ct.do?id=3399
[17] Grossman, J., Reid, P., & Morgan, R., (2001).Contributions of Academic Research to Industrial Performance in Five Industry Sectors, The Journal of Technology Transfer 26(1-2).
[18] Inkpen, A. (1996). Creating knowledge through collaboration, California Management Review, 39(1), 123 -140.
[19]. Laestadius, S. (2004). Innovation Management in the Knowledge Economy: Series on Technology Management, vol. 7; Ben Dankbaar (Ed); Imperial College Press, London, 2003, 371 pages (including index), hardcover, ISBN 1- 86094-359-4. Technovation, 24(7), 593-594.
[20] Le Houx, P. (2002). Une analyse critique de la valeur des technologies et des processus innovants peut- elle nous amener à concevoir de nouveaux instruments de regulation, Montreal: Université de Montreal.
[21] Leydesdorff, L. (2003). Communication and Knowledge: how is the knowledge base of an economy constructed? Paper presented at the 15th annual meeting of the society for the Advancement of socio-Economics, SASE, Aix en Provence, France.
[22] Leydesdorff, L., & Meyer, M. (2000). The Triple Helix of university-industry-government relations, Kluwer Academic Publishers B.V. , 58(2), pp 191-203
[23] Malhotra. (2003). Managing and Measuring Knowledge assets in the public sector, Retrieved 01.01.2007, 2007
[24] McDermott, R., & O’Dell, C. (2001). Overcoming cultural barriers to sharing knowledge, Journal of Knowledge Management, 5 (1), 76- 85.
[25] McIrnerney, C. (2002). Knowledge management and the dynamic nature of knowledge, Journal of the American Society fir Information Science and Technology, 53(12), pp 1009-1018.
[26] Nonaka, I., & Takeuchi, H. (1995). The Knowledge-Creating Company: How Japanese Companies Create the Dynamics of Innovation: New York:Oxford University Press.
[27] Pemberton, J. D., & Stonehouse, G. H. (1999). Learning and knowledge management in the intelligent organisation, Participation and empowerment: An International Journal, , 7(5), pp 131-144.
[28] Pemberton, J. D., & Stonehouse, G. H. (2000).Organizational learning and knowledge assets – an essential partnership, The Learning Organization, 7(4), pp 184-193.
[29] Pérez, P., M. , & Sánchez, M., A. (2003). The Development of University Spin-offs: Early Dynamics of Technology Transfer and Networking, Technovation, 2003, Vol. 23, No. 10, pp 823- 831., 23(10), pp 823-831.
[30] Polanyi, M. (1958). Personal knowledge, Chicago University Press.
[31] Richard, L. R. (2003). The Physician Culture and Resistance to Change Part I: Why Physicians Stick to the Status Quo, Retrieved 31/12/2006, 2006, from http://www.healthleadersmedia.com/view _ feature.cfm?content_id=43883
[32] Sandelin, J. (2003). Success Factors in University Technology Transfer through Patenting and Licensing, InnovationMatters, 15(15), 10.
[33] Sunassee, N. N., & Sewry, D. A. (2002). A Theoretical Framework for Knowledge Management Implementation, Paper presented at the SAICSIT (South African Institute of Computer Scientists and Information Technologists) Annual Conference.
[34] Todd R. Johnson, Jiajie, Z., Vimla L. Patel, Alla, K., Xiaozhou, T., Juliana, J. B., Danielle Paige, et al., The Role of Patient Safety in the Device Purchasing Process, Safety in Device Purchasing, 1, 341-352.
[35] Tyler, C. (2006). Single-Payer Health Care and Medical Innovation, Retrieved Januray, 2007, from http://economistsview.typepad.com/economistsview/2006/10/singlepayer_hea.html
[36] van Baalen, P., Bloemhof-Ruwaard, J., & van Heck, E. (2005). Knowledge Sharing in an Emerging Network of Practice: The Role of a Knowledge Portal, European Management Journal, 23(3), 300-314.