Feasibility of Active Ingredient (AI) development for new biocides in the EU
Biocides usage covers a vast industrial area and the history of biocide and other antimicrobial agent usage in various forms and applications dates back centuries. While the emerging EU regulations (BPD, REACH and others) strive to increase the safety and the eco-efficiency of chemical products and production processes, such changes may also create voids in the availability of current biocides due to outphasing. The present study evaluated the need for new Active Ingredient (AI) development. The feasibility of such development was explored, and the data of economic feasibility analysis shows that, contrary to general expectations, AI development can become profitable within certain economic boundaries.
Introduction
Biocides (antiseptics, disinfectants and preservatives) are usually broad spectrum chemical agents that inactivate micro-organisms (Russell, 2003; McDonnell and Russell, 1999) and their effects are highly concentration dependent (Russell and McDonnell, 2000). The history of biocide and other antimicrobial agent usage in various forms and applications dates back centuries (Russell, 2002). Microorganisms, although vital for our health and environment, may also pose a risk to human health and can cause serious problems and economic loss in various industries. Biocides are thus used to disinfect, to eliminate undesired organisms, preserve and conserve various products (Rasmussen et al., 1999). On the other hand, the desired properties of biocides include minimal toxicity and eco-toxicity, yet they need to have spectrum of activity and stability adapted to the application in question (Paulus, 1996).
Biocides are used in a variety of different products and applications, where the consequences of possible microbial contamination and spoilage range from being life-threatening, e.g. in pharmaceuticals (Zani et al., 1997) and in food (Raczek, 2005) to deterioration of product quality and product recall costs with negative implications to consumer choice e.g. cosmetics, skin care products and toiletries (Scholtyssek, 2005). Biocides are also used in the prevention of microbial spoilage of fuel and oilfield operations (Robbins and Levy, 2005; McIlwaine, 2005). Biocides are added to the formulations of polymer dispersions, mineral dispersions and paints (Gillatt, 2005; Schwarzentruber and Gane, 2005; Lindner, 2005). Biocides are also needed for the protection and preservation of plastics, textiles and leather (Dylingowsky and Hamel, 2005; Wypkema, 2005; Hauber, 2005). In the pulp and paper industry biocides play an important role in different processes where they are crucial especially in the prevention and controlling of biofilm formation (Corbel, 2005). An important biocide application is microbiological quality control in cooling water systems and in recreational water applications, such as swimming pools, spas and water amusement parks (Ludensky, 2005; Unhoch and Vore, 2005). In addition, biocides are used in the protection of industrial wood and water-mixed coolants (Williams, 2005; Siegert, 2005).
Accordingly, the usage of biocides covers a vast industrial area and applications thereof. In 2004 the world biocide demand was 5,300 million dollars from which 2,620 million dollars (49%) was used for preservatives, 1,685 million dollars (32%) for water treatment and 995 million dollars (19%) for disinfectants or for industrial processes. By 2009 the world biocide demand is estimated to grow to 6,880 million dollars (5.4% annual growth from 2004) and by 2014 to 9,050 million dollars (5.6% annual growth from 2009) (Freedonia, 2005). Currently, the biocides industry as a whole is governed by end-industry growth, technological developments, regulatory changes and the growing use of biocides as an aid to improve hygiene (Anon., 2007; Anon., 2008a). On the other hand, the industry and R&D of new biocides faces challenges because of the changing industrial and regulatory environment (Bruns et al., 2005). The registration costs of the EUwide Biocidal Products Directive including all toxicological tests have been estimated at 3-4 million euros (Bruns et al., 2005), which will not ease the existing stagnation of biocidal product development. Moreover, only limited efforts are being invested in the R&D of new Active Ingredients and many of the companies manufacturing biocides have focused on making new biocide combinations from existing Active Ingredients (AI). The term Active Ingredient (AI) is an even more precise term when referring to the actual compound which is responsible for the functional properties of the biocide. On the other hand, biocidal products may also be formulations of many Active Ingredients (Paulus, 2005; Bruns et al., 2005). In addition, the EU Biocidal Products Directive (BPD) 98/8/EC uses the term Active Substance when addressing Active Ingredients (Anon., 1998).
The BPD 98/8/EC addresses the placing of biocidal products on the EU market. The aim of the Directive is to enforce an authorization procedure based on a risk assessment for products containing biocidal active substance before placing the products on the market. The objective of BPD is also to remove barriers of trade between Member States and create a harmonised high degree of protection for people and the environment (Rasmussen and MacLellan, 2001). The BPD applies to 23 different product types listed in Annex V of the Directive but excludes some product types covered by other Community legislation, e.g. cosmetics (Matthews, 2002; Anon., 1998). The BPD entered into force May 1998 and 24 months was given to Member States to implement the directive. A 10-year period following the implementation deadline within the Member States was given to evaluate all active substances used in all products. After this period, active substances not found from the directive’s Annexes I, IA or IB must be withdrawn from the market for use as biocides as must also the products in which they are used (Rasmussen et al., 1999). The EC Commission Regulation of BPD has listed some 1,000 active substances from which only over 300 active substances and product types were included in the original review programme (Anon., 2003). The BPD regulation requires extensive testing of all biocidal products before they are registered for sale and the registration is required for both new and old biocides (Bruns et al., 2005).
Accordingly, emerging EU regulations (BPD, REACH and others) strive to increase the safety and the eco-efficiency of chemical products and production processes. However, it may be argued that such changes will create voids in the availability of current biocides, which may become out-phased. It therefore follows that the chemical industry is in need of new AI development in order to comply with new regulations and demands for increased environmental stewardship. Consequently, we have studied the economic feasibility of new AI development and the need for such development from an industry viewpoint. In order to address these questions, a profitability evaluation for a new AI was performed based on the net present value (NPV) and discounted payback period (DPP). In addition, data was collected by interviews in order to obtain an overview from different industries on needs and views with reference to biocides and new product development thereof.
Methods
Profitability evaluation using a sensitivity analysis
The basic measure of profitability is the net present value (NPV) of a new AI development. A development project should be carried out if the discounted net cash flows during the AI’s life cycle exceed the R&D investment, i.e. NPV is greater than zero. Our data is based on general estimates of development costs (Kähkönen and Nordström, 2009) and as well as on biocide demand (Freedonia 2005) but includes no information on specific competition and manufacturing operations. Therefore, NPV is estimated according to Equation 1.
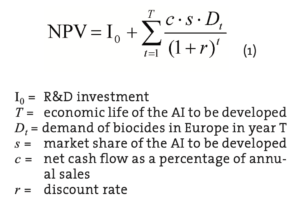
It should be noted that the net cash flow percentage (c) contains implicit assumption concerning operating profit margin, cash cycle of the operations and tax rate. Likewise, the market share depends on future pricing decisions concerning not only the AI but its rivals or substitutes.
Therefore, the purpose of the economic analysis is to explore the boundaries of economic feasibility. The approach is that of a sensitivity analysis or break-even analysis, to be more specific (Brealey and Myers, 2003). In a breakeven analysis, the analyst seeks for boundary values that make the projects NPV equal zero. In order to facilitate the comparison of scenarios, we also use discounted payback period (DPP) as an indicator. The discounted payback period points to the minimum economic life (T) that makes NPV positive.
Industry Interviews
Interview data was collected in 2008 and selected Finnish companies were chosen to represent various industries utilizing biocides. The interviewed Finnish companies manufactured 1) adhesives and related products; 2) pharmaceuticals; 3) cosmetics and skin care products; 4) polymer dispersions; and 5) biocides for the use of mainly pulp and paper industry. In addition, four companies operating in the paint industry were also included in the study but the data was collected by using a questionnaire form. The oral interviews were conducted in a semi-structured manner (a focused interview) (Hirsjärvi and Hurme, 1993; Tiittula and Ruusuvuori, 2005). All the interviews were recorded and documented in writing afterwards. Although the interviews dealt with three different themes (Table 1), only the answers concerning the focus area of the present study were analyzed.
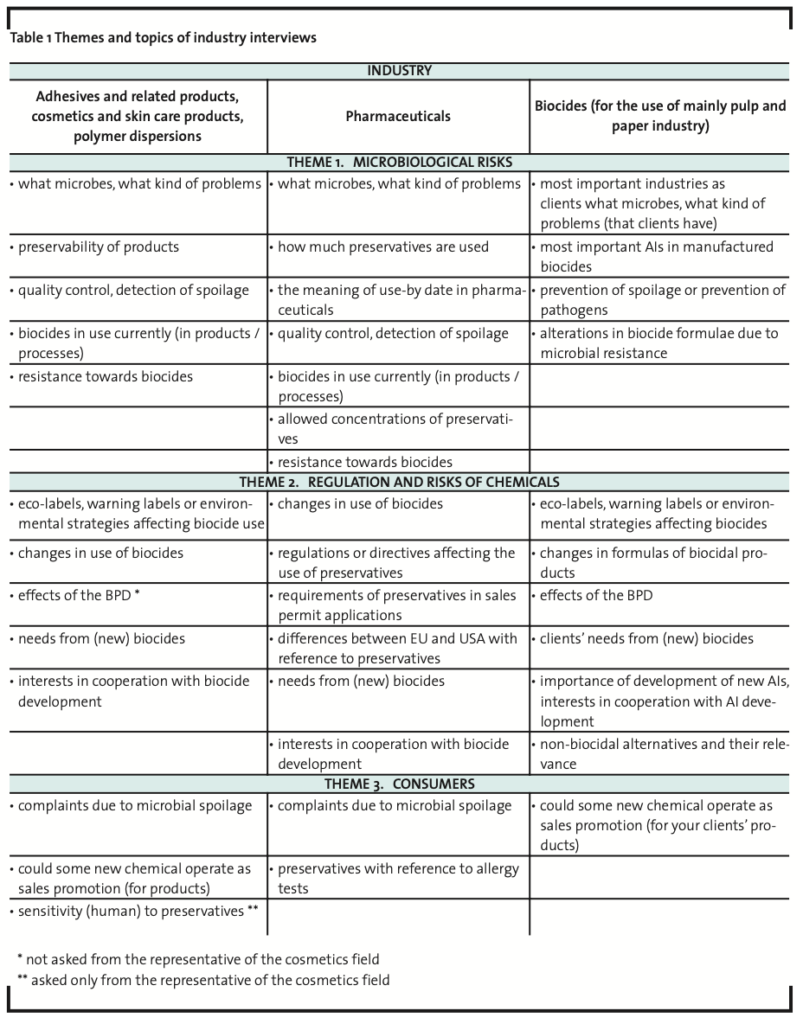
In addition to the interview data collected from the industries utilizing biocides, a representative from the Finnish Allergy and Asthma Federation was interviewed (Table 2). The interview on preservative allergies was also conducted in a semi structured manner (a focused interview) (Hirsjärvi and Hurme, 1993; Tiittula and Ruusuvuori, 2005) and the interview was recorded and documented in writing afterwards.
Results
Profitability of a new biocide
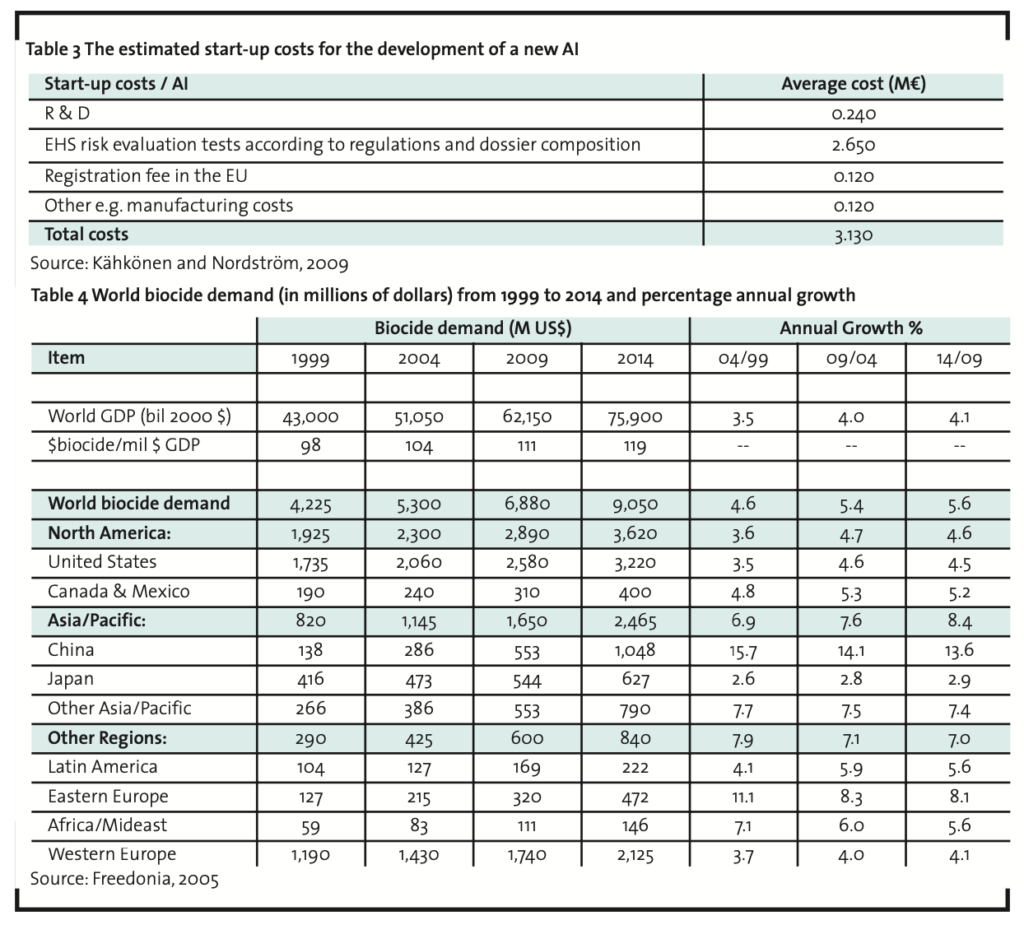
The basic parameters of the NPV model are R&D investment, demand and discount rate. After setting values for these parameters, we have sought the break-even values for market share and net cash flow percentage. Based on a previous study, the R&D investment is estimated to be € 3.13 million (Table 3).
The demand estimates are only for Europe and are based on the summed value of the demand in Western and Eastern Europe in 2009 (Table 4). According to the data in Table 4 the biocide demand in Europe as a whole in 2009 is 2,060 million dollars. Converted to euros (€ = US$ 1.3705 in 2007; Anon., 2008b) the demand for biocides in 2009 in Europe is 1,503 million euros1. The expected growth for the biocide demand of the world is estimated as 5.6% annually from 2009 until 2014. This growth estimate of the world biocide demand is used in the calculations although covering only Europe. The new AI is assumed to be on the market at the beginning of the year 2009 (t=1) and on the market for at least until 2030 (T=22). The estimated market life is in alignment with the long life cycles of the many currently used biocides. As there is no growth estimate beyond 2014 provided by Freedonia (2005) or any other public source, the calculations are based on an assumption of an annual growth percentage of 3.0 from 2015 until 2020 and 1.0 from 2021 to 2030.
The diffusion time of a new product into the market is not taken into account and therefore the market share is assumed to be gained immediately. The discount rate is assumed to be 10% throughout the life cycle. Discount rate and annual demand in euro represent nominal values.
Since the main purpose is to justify further investigations in AI development, we have searched for modest target levels of market share and net cash flow percentage. If one accepts a discounted payback period of 22 years and net cash flow percentage of 5%, the required market share is 0.36%. A market share of 0.18% would be sufficient if net cash flow percentage were 10%. The form of Equation 1 shows that the relationship between these two variables is linear ceteris paribus. If we assume a discount rate of 15%, the respective figures are (5%, 0.51%) and (10%, 0.26%). If we assume a more moderate market growth, namely a constant growth rate of 2.65%2, a discount rate of 10% and a net cash flow percentage of 5% would require a market share of 0.39%. Table 5 illustrates another scenario with the initial assumptions. Under more favourable conditions, NPV can be much greater than zero and the discounted payback period less than 10 years. As a conclusion, it can be stated that in order to become profitable in the biocide market in Europe, the minimum targeted market share should be approximately 0.4% for a new AI. This is consistent with a net sales target of 6 million euros for the first year.
Views of the industry and need for new biocides
The interviews of the present study indicated that industry representatives are concerned about the changes in legislation and consequent effects on available biocides. The interviewees operating in the business-tobusiness field highlighted that warning labels in products were not desired. REACH was thought to affect the availability and warning labelling or to increase the prices of the used raw materials. REACH was also seen as affecting companies via the required pre-registration demands. Only one interviewee estimated that the BPD would increase prices of biocides. This same interviewee also believed that no new AIs would be able to enter the biocide market due to the high costs of BPD for new AIs. In addition, eco-labels were also mentioned as affecting the use of biocides. Depending on the industry, preservatives such as formaldehyde (and formaldehyde releasing compounds) and parabens were named as controversial biocides. Formaldehyde was avoided or not used at all due to its toxicity, but also due to changed customer demands, the EU Water Framework Directive and due to environmental labels such as the Blue Angel (Der Blaue Engel). Representatives who mentioned these were from the adhesives and related products company and the polymer dispersions company. The industry representatives of the pharmaceuticals and cosmetics field stated that there is a trend to discontinue the use of parabens due to possible allergies and negative media hype. These examples indicate problems with currently used biocides and the lack of alternative biocides.
Properties needed for new biocides
Differing views were presented as to the kinds of properties a new biocide should ideally have. Only one interviewee stated that a new biocide should comply with certain standards so that use of such a biocide would not lead to demands for warning labels. Moreover, the biocide should be effective so that it could be used only in small amounts. On the contrary, however, another interviewee stated that it would be useful if a new biocide could be used in large amounts, but without warning labels. In general, interviewees were of the opinion that a new biocide should be highly effective, broad-spectrum and safe to use with no sensitization problems. In addition, the interviewees from the paint industry all agreed that there is a clear need for new dry film preservatives.
There also appears to be need for biocides that would function with a completely different mechanism compared to the biocides in use currently. Such a new biocide could be e.g. some natural raw material that would have biocidal attributes. It was also mentioned that a new biocide should also be suitable for many different kinds of products and have a good solubility. The company manufacturing biocides listed cost-effectiveness as the most important attribute in addition to the biocide being safe and rapidly degradable to harmless end-products.
A highly important issue that also arose in the interviews was a need for new biocide efficacy testing methods. Interviews of the paint industry indicated that the most important area for basic research on biocides is determination of biocide efficacy. In addition, the representative of the company manufacturing adhesives and related products stated that microbiological knowledge and business related to biocide efficacy and product preservability is still limited. More efficient and specific methods for biocide efficacy testing are thus called for. They are also crucial for the development of new biocides as giving a basis for the development process of a new AI.
In addition, also the non-biocidal solutions and alternatives to chemical biocides were discussed in the interviews. Although there was no direct question on non-biocidal means for microbial control, many of the interviewees mentioned non-biocidal alternatives during the interviews and the importance of phrases such as ‘preservative-free’ in advertising. The representative from the cosmetics field emphasized that there is an ongoing trend towards reducing the use of preservatives. This interviewee also stated that if the product can be marketed as ‘preservative-free’, it clearly adds value to the product. The representative from the pharmaceuticals field also mentioned this same trend and the possibility of using e.g. disposable packages or preserving packaging technology instead. This interviewee also mentioned that there is a trend towards disposable packages.
The Finnish Allergy and Asthma Federation interview revealed aspects of the sensitizing potential of many preservatives. The interviewee emphasized that even though the number of some allergies has increased during recent years, this should not be interpreted as a proliferation of allergies in general. Rather, the increase is due to increased knowledge on and diagnostics of allergies. Even though allergies have not increased, the interviewee mentioned that the so called ‘sensitivity markets’ have grown during the last few years especially in the cosmetics field. In new AI development the sensitizing aspect is therefore a crucial aspect to be taken into account as many preserved products are used on skin on a daily basis. On the other hand, industrial preserving methods should be environmentally friendly and should not be bio-accumulative.
Limitations of the study
The interviews conducted in this study represent a qualitative research method. A potential challenge in achieving validity in qualitative research is researcher bias, arising out of selective collection and recording of data, or from interpretation based on personal perspectives (Johnson, 1997). This also applies to the present study as the interviewed sample was small and consisted of industry representatives who were not selected through random sampling. However, although the number of interviewees is rather limited, it is to be noted that the interviewees represented companies with a vast range of biocide application needs. Moreover, at this point the aim of our study was only to shed some preliminary insight into the needs and views of the industry and whether there is any indication that such views take into account the cost of new biocide/AI development.
In this study, the calculations were performed in order to give a rough estimate rather than precise figures. This was due to the limited initial information and consequently the many assumptions that had to be made. Moreover, the conversion of the initial numeric data from dollars to euros clearly is dependent on the used exchange rate and therefore affects the value of the turnover and cash flow estimates. The calculations were performed based on the biocide market size estimates published in 2005 as this was the newest available information. However, the current financial situation clearly has an influence and more recent market growth assumptions would presumably be different and more moderate. The financial situation was, however, taken into consideration by making the longer term growth assumptions rather on the low side in order not to overestimate the market size. In addition, the calculations were performed using a sensitivity analysis where various different estimates of the cash flows were calculated.
The calculations of this study focused only on the assessment of the net present value and the discounted payback period based on the basic initial costs which composed mainly of the registration fee, EHS risk evaluation costs and moderate R&D costs. However, developing an AI with the attributes specifically wished for by the industry representatives might increase the estimated R&D costs even heavily. But as the estimation of these specific R&D costs would be highly inaccurate and difficult, the calculations were carried out using a very moderate estimate of the startup costs. Clearly, these calculations can be made more accurate when more information on the development and other costs is available. In addition, the calculations in this study do not take into account those R&D costs and development processes which do not lead to a desired outcome. In further studies when more initial information is available, different and more complex calculation models can be used for profitability evaluation.
Discussion
While the changing industrial environment of the biocide market and the high registration costs of the BPD are not generally seen as an incentive for developing new AIs, the industry needs should be seen as one instead. The conducted interviews echo the opinions of industries and speak on behalf of new AI development. In addition, the limited number of available biocides can be seen as a problem, especially if the number of the AIs in use currently decreases due to the BPD as has been speculated (Bruns et al., 2005; Chapman, 2003). The EC Commission Regulation of BPD has listed some 1000 active substances from which only over 300 active substances and product types were included in the review programme (Anon., 2003).
The data of the present study does not support the general opinion that development of new AIs would be economically unfeasible. Rather, it is evident that the payback time for new developments will increase, but as the present study has shown, the market shares for profitable operating in the European market are not overwhelming. If one accepts a discounted payback period of 22 years and net cash flow percentage of 5%, the required market share is 0.36%. Therefore, in order to become profitable in the European market, the minimum targeted market share should be approximately 0.4% which is consistent with a net sales target of 6 million euros for the first year. Under more favourable conditions, NPV can be much greater than zero and the discounted payback period less than 10 years.
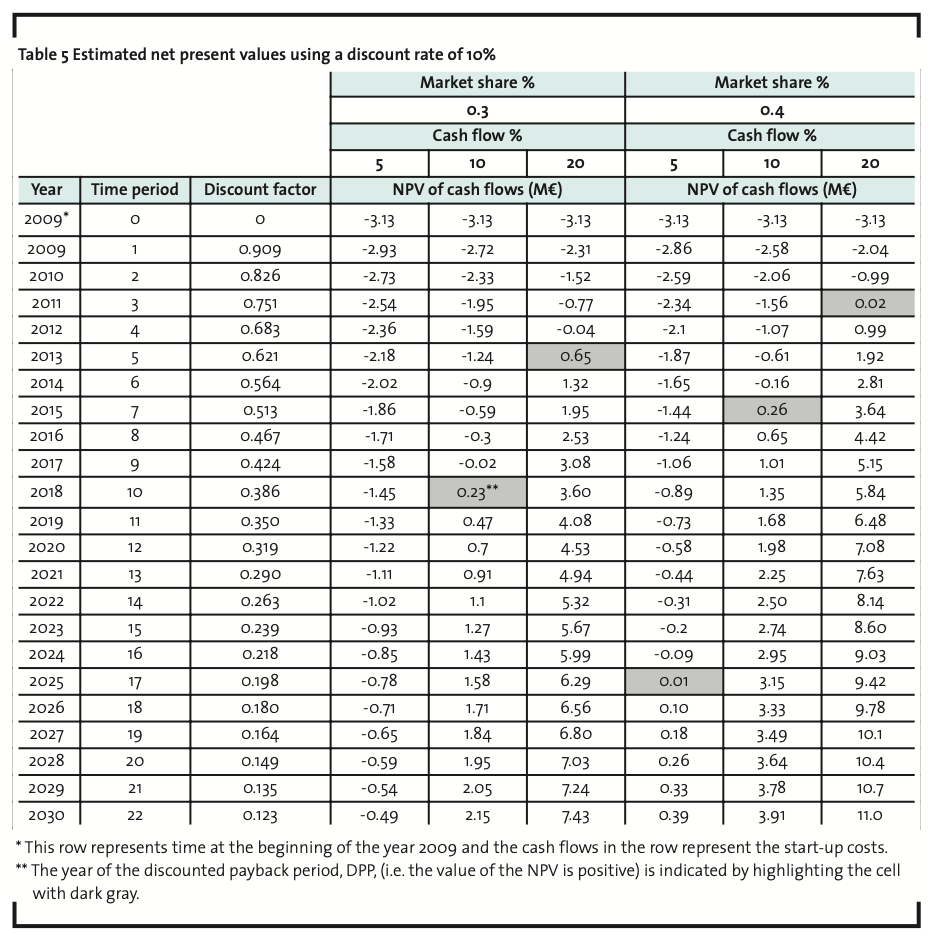
A market share of 0.4% can be seen as an achievable one especially as some over 300 AIs are currently included in the review programme of the BPD. Hypothetically, if all 300 AIs would have an equal market share it would equal 0.33%. The market share limit calculated in the present study is somewhat higher but still in the same range. Thus for an efficient, new product, a market share of 0.4% appears realistic. In addition, the demand for new, efficient biocides can be argued to increase as the number of currently used AIs decreases due to implementation of the BPD which reduces the amount of alternative AIs.
In addition to chemical preservatives, there is also interest in alternative preservation methods. However, it seems highly unlikely or even impossible that these would rule out the chemical methods entirely. Rather, the development of non-chemical methods is extremely important, but in many cases these methods can be utilized alongside or in addition to chemicals. As an example, the development of preserving package technologies is a fertile field, whereas increasing the amount of disposable packages vs. chemicals should be carefully evaluated. Disposable packages increase the amount of waste and therefore are not necessarily the only sustainable alternative for chemicals.
As the BPD requires registration of both new and old biocides (Bruns et al., 2005) also existing AIs in the market will require investments. Consequently, this would argue on behalf of the importance of new AI development as costs of registration are inevitable if the aim is to stay in the biocide market. In addition, as the biocide market may become more stagnated, a new AI may rapidly gain visibility and the producer of a new AI may become recognized as a technology leader. On the other hand, if new AIs are not developed, then it may be wise for companies to include a broader range of control of biodegradation, including also non-chemical means of control. Controversially, however, the increasing demand for non-chemical, ecologically more acceptable means for control may become a barrier to entrance of new AIs. On the other hand, it is unlikely, that non-chemical alternatives solely would be adequate for all purposes in the near future, whereas a new AI together with non-chemical means could perhaps offer a highly versatile product portfolio for a biocide producer.
Although one of the goals of the emerging regulatory framework for chemicals within the EU strives to boost innovation within the Community, it is evident that the implementation of regulatory initiatives may also pose a possible barrier for new AI development. Evidently, the competitiveness of the EU market is inherently tied also to international trade and harmonisation of regulatory initiatives worldwide. If harmonisation of legislation does not support the competitiveness of the EU as intended by the Community regulatory framework, unexpected challenges for AI development might occur, as global requirements will differ and increase the cost of a “universally” acceptable new AI.
New AI development should be based on a standardized and reliable protocol for biocide efficacy testing. However, at the moment efficacy testing methods for biocides vary according to the industry application and can also be seen as a barrier for new products entering the market. The pharmaceuticals industry is an example of an industry which has a clearly defined testing protocol for efficacy testing and acceptance criteria (Meyer et al., 2007). In many other industries various different efficacy testing methods and protocols exist (Gillatt, 1991; Heinken, 2000). Therefore, biocide efficacy testing is clearly an area that needs to be further studied and developed in order for the efficacy of new AIs to be verifiable.
In conclusion, development of new AIs is technologically and economically feasible. Clearly also non-chemical alternatives for preservation should be considered, possibly in conjunction with chemical biocides development. Alternative means of microbial control are often less toxic to the user, consumer and the environment, but limited as to the range of applications where such methods can be used. On the other hand, chemical biocides clearly suffer from toxicity, which, however, is inherent to their activity and required efficacy profile. Consequently, the challenge for biocides and control of biodeterioration in the future is ingrained into formulating the “non-toxic poison” for which the price-tag remains to be determined.
REFERENCES
Anonymous (2007): Global Biocides Report – 2007; Summary, available at http://www.researchandmarkets. com/reportinfo.asp?report_id=448961&t=e&cat_ id=, accessed 11 June 2008.
Anonymous (2008a): Global Biocides Report, available athttp://www.biocide-information.com /index.php?option=com_content&task=view&id=510 4&Itemid=35, accessed 11 June 2008.
Anonymous (2008b): Suomen Pankki – Valuuttakurssit; vuoden keskiarvo, available at http://www. suomenpankki.fi/Stats/default.aspx?r=%2ftilastot%2fvaluutta kurssit%2fvaluuttakurssit _short_fi, accessed 10 December 2008.
Anonymous (2003): Commission Regulation (EC) No 2032/2003 of 4 November 2003 on the second phase of the 10-year programme referred to in Article 16(2) of Directive 98/8/EC of the European Parliament and the Council concerning the placing of biocidal products on the market, and amending Regulation (EC) No 1896/2000, L307.
Anonymous (1998): Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market, OJ L123.
Brealey, R. A. and Myers, S. C. (2003): Principles of Corporate Finance, 7th ed., McGraw Hill Irwin, Boston, pp. 1-150.
Bruns, R., Kaulen, J., Kretschik, O., Kugler, M. and Uhr, H. (2005): R&D in material protection: new biocides, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 25-46.
Chapman, J. S. (2003): Biocide resistance mechanisms, International Biodeterioration & Biodegradation, 51, pp. 133-138.
Corbel, G. (2005): Pulp & paper, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 377-410.
Dylingowsky, P. J. and Hamel, R. G. (2005): Microbial degradation of plastics, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 325-346.
Freedonia (business research company) (2005): World Biocides to 2009, Industry study.
Gillatt, J. (1991): Methods for the Efficacy Testing of Industrial Biocides – 1. Evaluation of Wet-State Preserva
tives, International Biodeterioration, 27, pp. 383-394. Gillatt, J. W. (2005): The microbial spoilage of polymer dispersion and its prevention, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 219-250.
Hauber, C. (2005): Microbicide applications in the leather industry, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 317-324.
Heinken, B. (2000): Microbiological Quality Management for Waterborne Coatings, Färg och Lack Scandinavia, 46, pp. 4-11.
Hirsjärvi, S. and Hurme, H. (1993): Teemahaastattelu, 6thed., Yliopistopaino, Helsinki.
Johnson, R. B. (1997): Examining the validity structure of qualitative research, Education, 118, pp. 282-292.
Kähkönen, E. and Nordström, K. (2009): New Biocides Development: Are Safer Biocides Economically Feasible?, (working paper).
Lindner, W. (2005): Surface coatings, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Mate
rials – A Handbook, Springer, New York, pp. 347-376.
Ludensky, M. (2005): Microbiological control in cooling water systems, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 121-140.
McDonnell, G. and Russell, A. D. (1999): Antiseptics and Disinfectants: Activity, Action, and Resistance, Clinical Microbiology Reviews, 12, pp. 147-179.
Matthews, I. (2002): Global preservatives, Global Cosmetic Industry, 170 (11), pp. 62-64.
McIlwaine, D. B. (2005): Oilfield application for biocides, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 157-176.
Meyer, B. K., Ni, A., Hu, B. and Shi, L. (2007): Antimicrobial Preservative Use in Parenteral Products: Past and Present, Journal of Pharmaceutical Sciences, 96, pp. 3155-3167.
Paulus, W. (1996): Biocides – Mode of action, in: Heitz, E., Flemming, H.-C. and Sand, W. (ed.), Microbially Influenced Corrosion of Materials, Springer, Berlin, pp. 105-120.
Paulus, W. (2005): Introduction to microbicides, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 3-8.
Raczek, N. N. (2005): Food and beverage preservation, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 287-304.
Rasmussen, K., Chemin, P. and Haastrup, P. (1999): Regulatory requirements for biocides on the market in the European Union according to Directive 98/8/EC, Journal of Hazardous Materials, A67, pp. 237-251.
Rasmussen, K. and MacLellan, M. A. (2001): The control of active substances used in biocides in the European Union by means of a review regulation, Environmental Science & Policy, 4, pp. 137-146.
Robbins, J. A. and Levy, R. (2005): A review of the microbiological degradation of fuel, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 177-202.
Russell, A. D. (2002): Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria, Journal of Applied Microbiology Symposium Supplement, 92, pp. 121-135.
Russell, A. D. (2003): Similarities and differences in the responses of microorganisms to biocides, Journal of Antimicrobial Chemotherapy, 52, pp. 750-763.
Russell, A. D. and McDonnell, G. (2000): Concentration: a major factor in studying biocidal action, Journal of Hospital Infection, 44, pp. 1-3.
Scholtyssek, R. (2005): Protection of cosmetics and toiletries, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 263-286.
Schwarzentruber, P. and Gane, P. A. C. (2005): Application of microbicides for the storage protection of mineral dispersions, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 251-262.
Siegert, W. (2005): Microbicides for coolants, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 203-218.
Tiittula, L. and Ruusuvuori, J. (2005): Johdanto, in: Ruusuvuori, J. and Tiittula, L. (ed.), Haastattelu – Tutkimus, tilanteet ja vuorovaikutus, Vastapaino, Tampere, pp. 9-21.
Unhoch, M. J. and Vore, R. D. (2005): Recreational water treatment biocides, in: Paulus, W., Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 141-156.
Williams, G. R. (2005): Industrial wood protection, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 419-439.
Wypkema, A. W. (2005): Microbicides for the protection of textiles, in: Paulus, W. (ed.), Directory of Microbicides for the Protection of Materials – A Handbook, Springer, New York, pp. 411-418.
Zani, F., Minutello, A., Maggi, L., Santi, P. and Mazza, P. (1997): Evaluation of preservative effectiveness in pharmaceutical products: the use of a wild strain of Pseudomonas cepacia, Journal of Applied Microbiology; 83, pp. 322-326.