Options for CO₂-neutral production of bulk chemicals
Abstract
The rising utilization of non-fossil-based raw materials in the chemical industry initiates the transformation path to a more sustainable and less greenhouse gas (GHG) emission-intensive way of producing consumer goods. In this review, the focus of alternative, large-scale synthesis routes for bulk chemicals by the example of 1,4-butanediol (BDO) and formaldehyde from fossil and non-fossil feedstocks-based production processes are discussed. Furthermore, options to lower GHG emissions from scope 1-3 in the corresponding examples are presented and regional and regulatory aspects are discussed. Finally, the demands and needs for sustainable production of BDO and formaldehyde are shown research-, technology-, feedstock-, and regulatory-wise.
1 Introduction
With climate change, the damage caused by greenhouse gases not only has a global ecological dimension. For operators of emission-intensive chemical plants in Europe, greenhouse gases are now an increasingly noticeable economic burden. Since the European Emissions Trading Scheme came into force, emissions allowances have had to be purchased. The cost of these allowances has been rising for several years and is expected to rise further. Especially emissions from power generation and chemical production (scope 1, 2) have so far been subject to the trading system. These emissions can be reduced by using emission-free energy sources, the capture of carbon dioxide, and its subsequent long-term storage or use as a carbon source.
The emission potential of purchased raw materials (the so-called raw material rucksack) and the emissions that arise during the use and disposal of the resulting products (scope 3) are not subject to the emissions trading system. In the case of organic chemical products, this is primarily carbon dioxide, which is produced, for example, during the combustion of carbon-containing products after use. However, it cannot be ruled out that, with the accelerating climate change, the political framework will change in such a way that these emissions will also be covered and thus cause further emission costs. There are several ways to avoid these possible costs. One of them is to switch from fossil to renewable, vegetable carbon sources. Their carbon has been removed from the atmosphere through the natural carbon cycle, i.e. the photosynthetic binding of carbon dioxide in biomass. Therefore, the emission from products made from them does not increase the concentration in the atmosphere and is considered climate neutral. Another option is to create a technical carbon cycle. Methane, carbon dioxide, and carbon monoxide can serve as industrial carbon sources and thus close the carbon cycle.
In the following, scope 1-3 emissions of the chemical industry are analyzed using concrete company examples. Subsequently, options for reducing these emissions are presented by the example of butanediol and formaldehyde.
2 Background
2.1 Scope 1-3 emission sources and volumes in chemical production
Since 2017 the market price for CO2 European emission allowances has risen significantly from 5 to 38.5 EUR/t in February 2021, thus, emitting CO2 and other greenhouse gases becomes more and more costly. Consequently, major companies have announced that they intend to operate their sites in a climate-neutral manner within a reasonable period of time. As examples table 1 quotes published statements of three German chemical companies. They have been selected as model examples for companies active on different stages of the chemical value chain. According to its published presentation, BASF (2019) concentrates on the production of petrochemicals, monomers, intermediates, and heterogeneous catalysts, Evonik (2021) on specialty chemistry, and Henkel (2021) on adhesive technologies, beauty care, and laundry & home care. With these statements, BASF can be seen as a representative of the early stages of the value chain in high volume bulk and basic chemistry, Evonik as a supplier of medium volume specialty chemistry in the middle stages of the value chain, and Henkel rather as a producer and purchaser of medium to small volume specialty and fine chemistry to formulate consumer products. In the following, the influence this positioning can have on the corporate strategy towards climate neutrality by mitigation of greenhouse gas emissions and the contribution of biobased raw materials will be investigated.
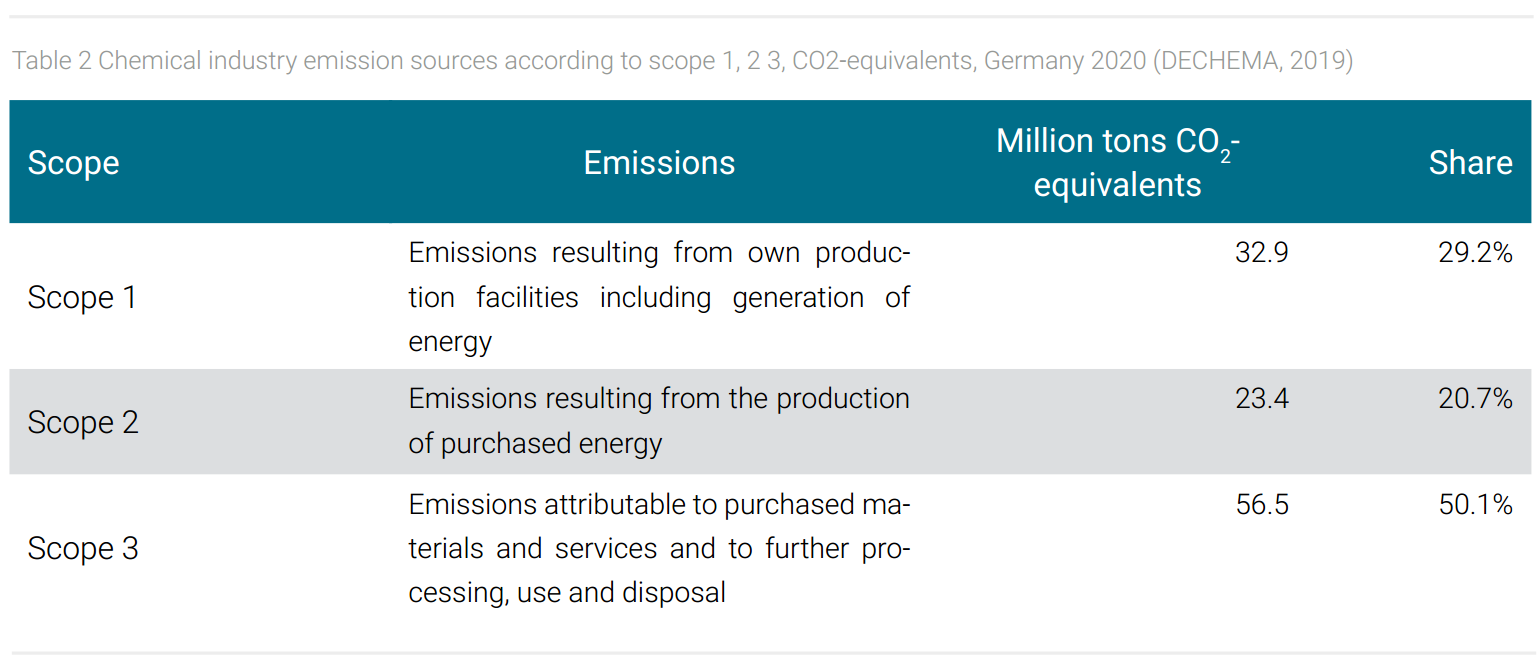
The sources of emissions in chemical production are manifold. They include emissions from energy generation for the chemical processes, from emissions from purchased energy, from emitted by-product greenhouse gases from chemical reactions, and from the downstream value chain (i.e. waste, transport, use, and disposal of products). These emissions are systematically defined according to scope 1, 2, 3 (tab. 2). In 2020, the different emissions for the German chemical industry, which will be presented below as a model example, amount to a total of 112.8 million tons of CO2 equivalents (DECHEMA, 2019). The data show that over the entire industry covered by the ETS, on average about half of the emissions are caused by chemical production processes (scope 1, 2) of which roughly one third are emissions of by-products of chemical reactions and two-thirds are emissions from the generation of process energies (scope 1, 2) (McKinsey, 2007). The other half contains, inter alia, the emission potential of the chemicals produced (tab. 2).
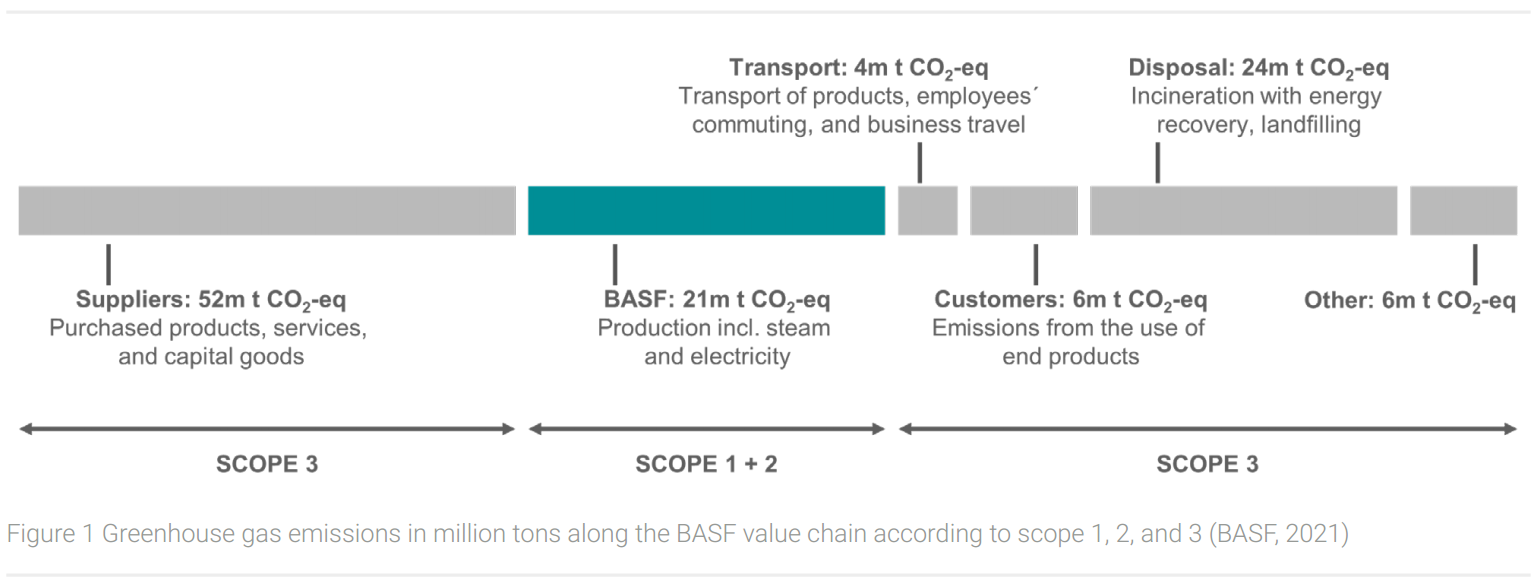
As a concrete example of a leading chemical company, BASF’s greenhouse gas balance for the year 2020 will be presented here. The company has been publishing a comprehensive greenhouse gas balance sheet (Figure 1) in accordance with the GHG Protocol Corporate Accounting and Reporting Standard since 2008 (BASF, 2021; World Resources Institute, 2021).
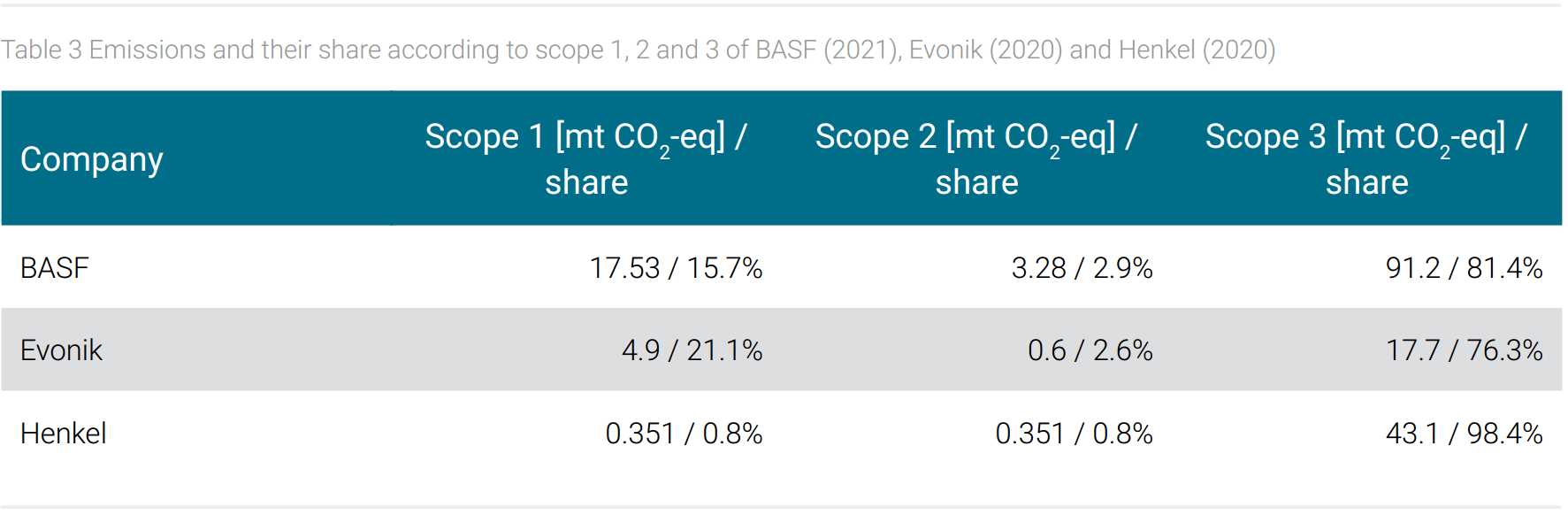
Table 3 shows the share of emissions according to scope 1, 2, and 3 for BASF, for Evonik, and Henkel. The different shares of energy-related emissions scope 1 and 2 of the three companies under consideration are striking. In fact, it seems the different energy-related emissions, respectively energy consumption is reflected in the companies’ product range. Plausibly, it can be assumed that BASF’s and Evonik´s comparatively high energy-related emissions are due to the energy-intensive basic chemical processes that are carried out there. The lowest energy consumption in this comparison is recorded by Henkel with its specialty chemicals end products geared to consumer applications. This interpretation is supported by a study that reports 80% of the energy-related emissions consumption of the German chemical industry to basic chemicals (World Resources Institute, 2021).
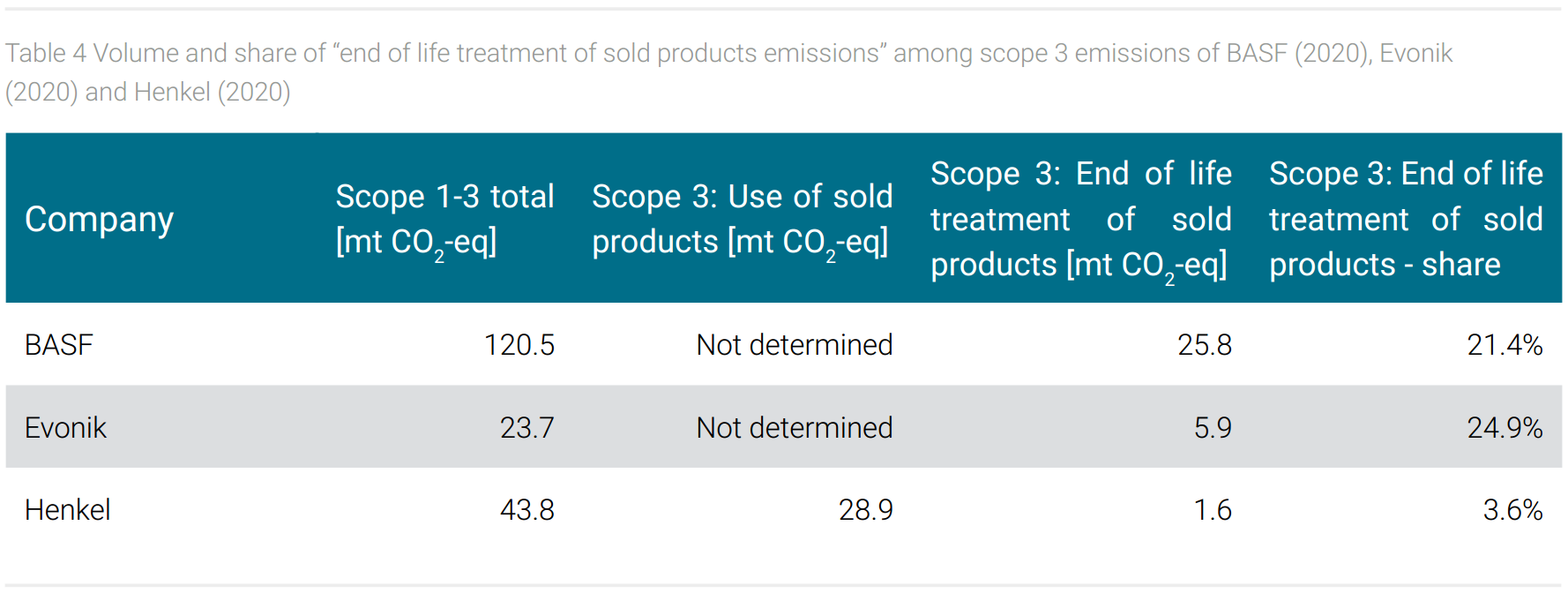
The product range of the three companies also seems to leave an imprint in the share of “end of life treatment of sold products” emissions (scope 3). For example, the monomers offered by BASF are processed by customers into a final plastic product that can be disposed of by different methods, one of which is energy recovery by incineration. The release of CO2 from incineration, the CO2 emission potential is included in this category. A personal care product from Henkel applied to the skin, on the other hand, is not disposed of in this way but is consumed with its application; i.e. the CO2 emission potential is not recorded as “end of life treatment” but as “use of sold products”. Table 4 shows that BASF and Evonik with their products to be delivered into the chemical value chain for further processing but not into consumer markets therefore do not report “use of sold products” emissions. The “end of life treatment” emission potential of these companies reaches a share of 21% and 25% among total reported emissions. For Henkel, this share is much lower at less than 2%, but the emissions caused by the “use of sold products” are reported at 66% (tab. 4).
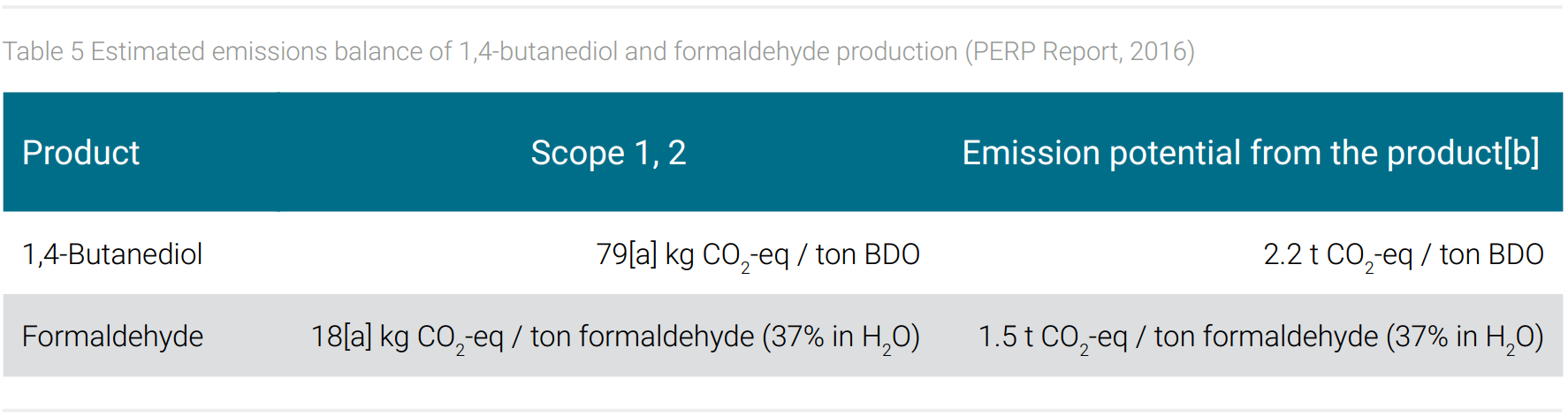
The product spectrum of a company, which can range from bulk to specialty and fine chemistry, thus influences the energy and product-related emissions scope 1-3. As already mentioned, in the context of emissions from chemical processing bulk chemicals are of particular interest because of their large production volume. Table 5, therefore, gives an example of the emissions from the production of formaldehyde and butanediol. Both chemicals are repeatedly discussed below as model examples. They were selected for BASF’s product portfolio because they fulfill the following, criteria of an important chemical intermediate: High production volumes, worldwide demand in several industrial sectors as they are mostly used as plastics monomers, high potential to replace their fossil-based synthesis route by a sustainable one. In the following, the possibilities to reduce the emissions that can be attributed to both chemicals will be presented and discussed. To characterize the exemplary products BDO and Formaldehyde, their specific scope 1 and 2 emissions from electric power demand in large scale production and the emission potential of CO2 from the chemical themselves (part of scope 3 emissions) are listed in table 5.
3 Discussion
3.1 Options to reduce scope 1-3 emissions
The scope 1 and 2 emissions reported come from fossil sources because the ETS only covers those emissions. Therefore, one way to reduce scope 1 and 2 emissions significantly is to switch to emission-free energies (hydropower, photovoltaics, wind energy, geothermal energy, nuclear energy). Henkel for example announces to switch to renewable energies (tab. 1). Bioenergy is also an option because its CO2 emissions are considered climate-neutral and therefore not priced by the ETS. Additionally, BASF´s Verbund concept, which is applied at all major sites, makes an important contribution to energy efficiency by linking production and energy demand. Waste heat from production processes is captured to be used as energy in other production plants. Because of the Energy Verbund, BASF saved in 2019 around 19.2 million MWh per year, equal to an annual reduction in CO2 emissions of 3.9 million metric tons (BASF, 2020).
3.1.1 Scope 1 and 2: CCS
Large CO2 flows are in principle suitable for capture and long-term storage (carbon capture and storage; CCS). In this way, emissions into the atmosphere are avoided by creating a CO2 sink as defined by the Paris Climate Convention.
The economical features of these processes are that they can save ETS allowances, but otherwise, they do not generate value.
Alternatively, CO2 can be captured and stored biologically in the form of biomass, for example in tree wood (Nijnik, 2010). A recent study reports that afforestation could contribute significantly to the greenhouse gas reductions required by 2030.(Austin et al., 2020) However, there are economical costs of land use and the ecological impact of indirect land-use change to be considered, and it must be ensured that this wood is not used in an emissions-releasing manner in the long term.
3.1.2 Scope 1 and 2: CCU
The situation is different for sinks that store CO2 emissions through utilization (carbon capture and utilization; CCU) as technical gas and as carbon source in chemical and biotechnological catalysis including photosynthesis.
Pure CO2 is required by the food and beverage industry and the pharmaceutical sector as a product component (beverages), and protective gas in packaging (Deerberg et al., 2020). In these fields most important by volume are the food and beverages applications consuming globally more than 31 mt (2018). However, these applications are no real option for CO2 sinks as the gas is released with the use of the products.
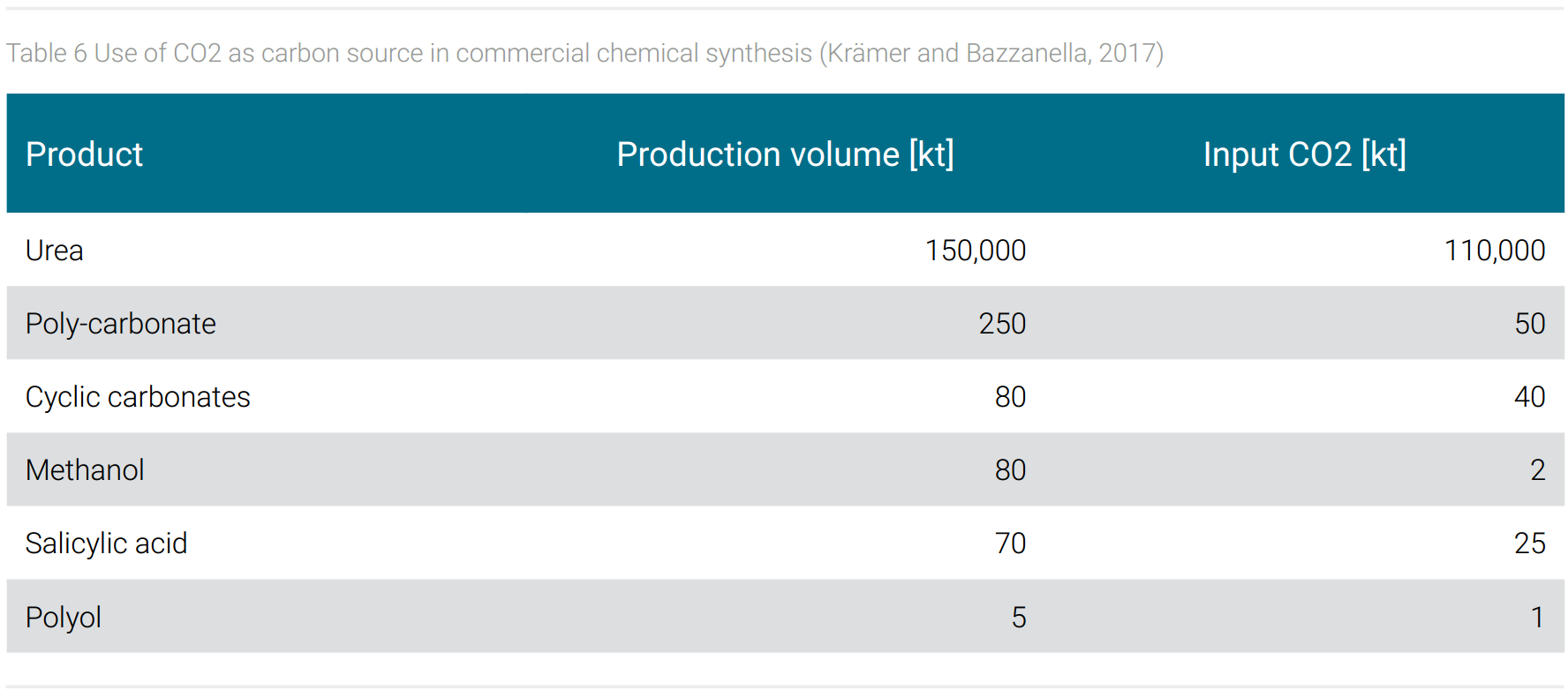
Established is the use of CO2 as carbon source in chemical synthesis. In Germany, for example, 75% of the CO2 produced during ammonia synthesis is captured and used, among other processes, in the production of urea and methanol (Krämer and Bazzanella, 2017). More commercial examples, their global production volume, and global CO2 input are shown in table 6.
Around 120 million tons of CO2 are used worldwide in the production of basic chemicals and intermediates. The carbon contained in these intermediates remains bound as long as the end products made from them are in use.
3.1.3 Scope 3: CCS and CCU
Scope 3 emissions originate from business and administration activities, transport of sold products to customers, further processing along the value-added chains, and the disposal of consumer products.
The use of emission-free power and fuels such as electricity, hydrogen, or e-fuels is an obvious way of reducing business, administration, and transport-related emissions. Because of the large number of small-volume emission sources like buildings and transport units, neither CCS nor CCU is an option here. This also applies to many installations at processing plants, unless they reach a size that is covered by the ETS itself.
The situation is different for emissions from the disposal of sold end products. Especially in urban areas, large volumes of waste are produced in a limited area and are collected centrally by municipal waste management authorities. In Germany and other countries in Western Europe, a significant share of municipal waste is recovered for energy (Scarlat et al., 2019) i.e. by its incineration. The resulting CO2 emissions are emitted into the atmosphere but could theoretically also be subject to CCS. However, the necessary infrastructure is not available, and it must be assumed that social acceptance of geological storage of CO2 in settlement areas will be difficult or even impossible to achieve (Tcvetkov et al., 2019). On the other hand, if small-scale CCU technologies would be available, the utilization of waste management emissions is an option to be discussed further below.
3.1.4 Scope 3: Switch to biogenic feedstock
One way to make the end-of-life share of scope 3 emissions more climate-friendly would be to change the raw material base of chemical products from fossil to renewable carbon sources. Biological carbon sources, on the other hand, contain carbon that was removed from the atmosphere by the photosynthetic carbon cycle and is therefore considered climate neutral in balance terms. Although the production of biomass is not entirely without greenhouse gas emissions, the release of this carbon does not generally increase the atmospheric CO2 concentration. Biological carbon sources thus offer a largely climate-neutral alternative to fossil raw materials, both for energy and material recycling.
The status of bio-based raw materials in the chemical industry is presented in the next section.
3.2 Status of biogenic carbon sources and chemicals
3.2.1 Biogenic raw materials
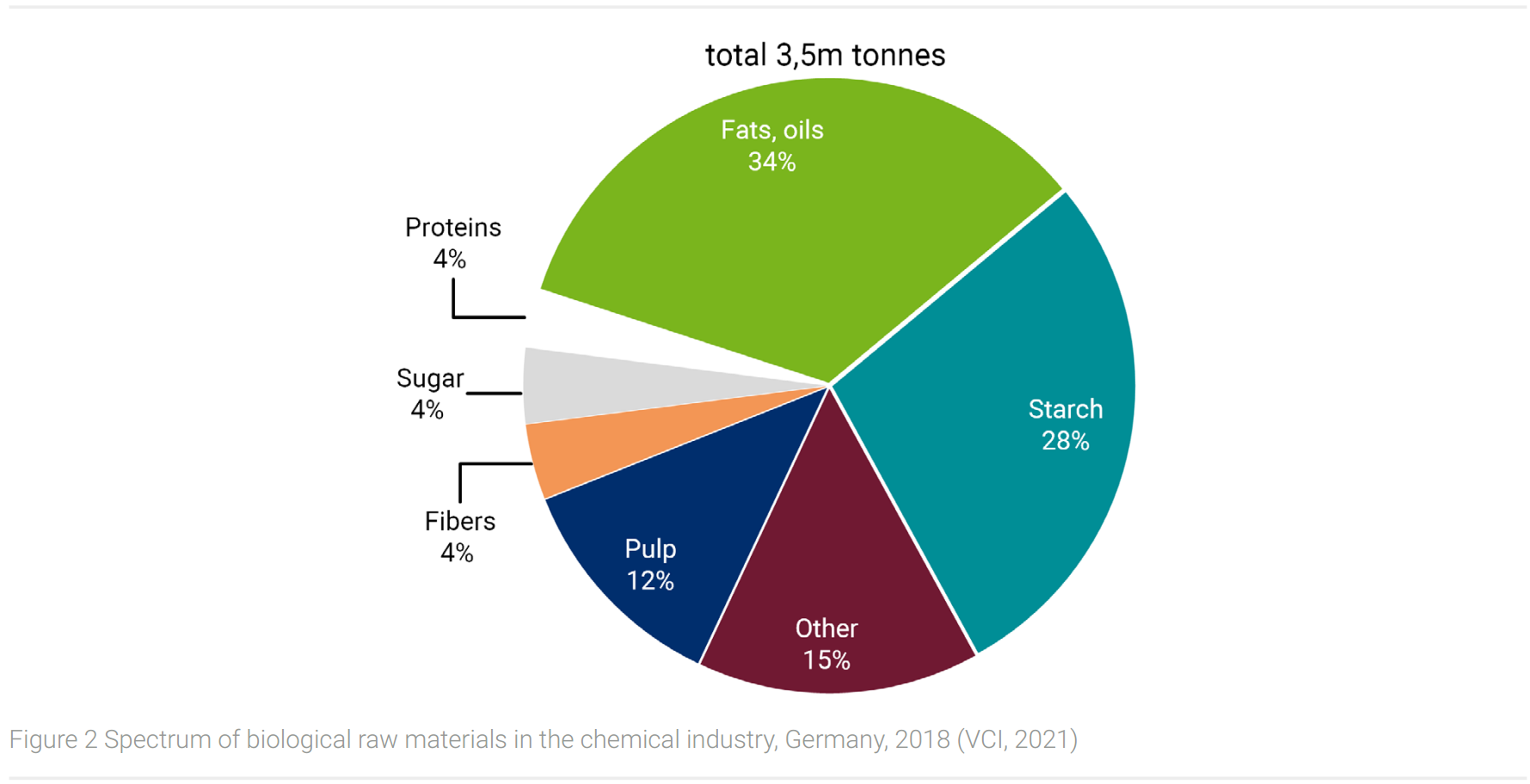
In the EU, the share of biogenic raw materials for chemical use is currently 10%, (Parasi and Ronzon, 2016) respectively 13% in the German chemical industry in 2018 (VCI, 2021). The spectrum of biological raw materials is shown in Figure 2.
At 34%, fats and oils have the largest share, followed by sugar and starch (32%). These, as well as pulp, fibers, and protein, represent fractions of the biomass of oil plants (fats and oil, protein), sugar and starch plants (sugar, starch), wood (pulp), and fiber plants (natural fibers). These are the raw materials for bio-based products listed in table 7.
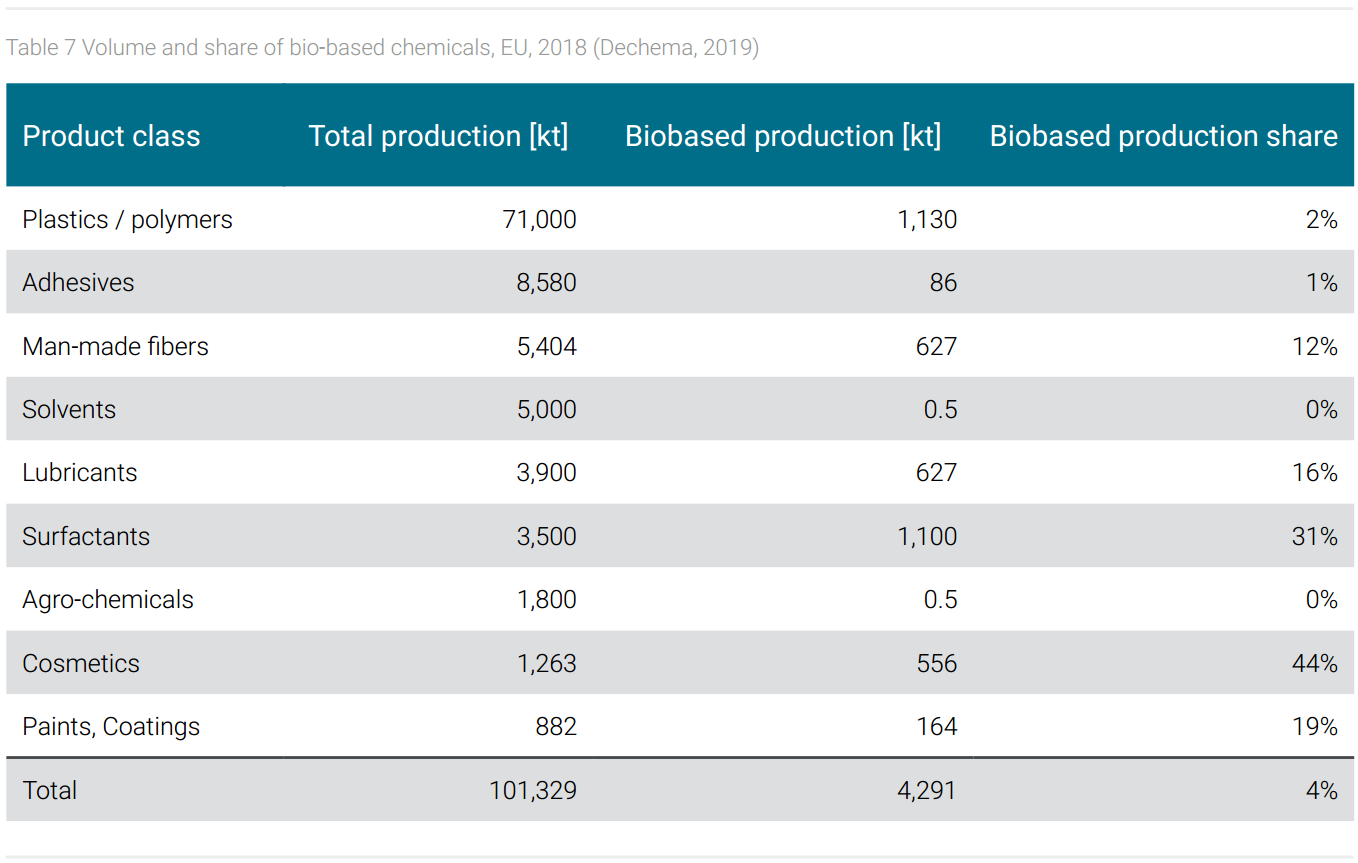
Amongst the bio-based chemicals shown in table 7, surfactants and cosmetics are the most important ones in terms of share, followed by lubricants, paints, and coatings. These products are classified as fine and specialty chemicals, which account for a comparatively small share of volume in the entire chemical portfolio. Their market success is based on a combination of technical performance and of customer expectations. The specific performance of lubricants, paints, and coatings depends in part on the properties of the raw materials, whereby, for example, natural fatty acids have advantages over synthetic fatty acids in terms of the proper chain length. In terms of customer expectations, surfactants and cosmetics meet a market that basically prefers biogenic products.
From the point of view of scope 3 emission reduction, it would be advantageous if large-volume basic chemicals and monomers were also produced on a bio-based basis. In fact, among bio-based chemicals, biopolymers are most relevant by volume. In the biopolymer market, PLA (polylactic acid) and PBAT (polybutyleneadiptate-terephtalate) are leading with 19%, respectively 14% (FNR, 2021). However, as shown in table 7, the share of biopolymers compared to total polymer production is only 2% in Europe, globally even smaller.
The reason lies often in significant cost disadvantages. In 2016, the costs for PLA and PBAT were estimated at 2,000 respectively 3,500 EUR/t; 50-100% more than the fossil-based alternatives (van den Oever et al., 2017). These additional costs are partly due to a lack of technological maturity and insufficient economies of scale but are also due to the basic properties of bio-based raw materials. Biomass has a lower carbon density compared to fossil raw materials. While natural gas and crude oil contain 75-87% carbon, dry biomass offers only about 50% of the sought-after element. On the other hand, the carbon yield of biotechnological processes is much lower than that of synthetic processes based on fossil carbon sources. The reason for this is that biological whole-cell catalysts extract energy for bio-catalysis from the raw material and the energy metabolism leads to the emission of CO2, thus the loss of carbon. In addition, unavoidable by-products are created, and part of the raw material is consumed to build the biomass of the biocatalyst. The low carbon density of feedstock and carbon losses in processing explain why the bio-feedstock share of 10% results in the bio-product share of only 4%.
The example of bio-1,4-butanediol, which is produced based on sugar, demonstrates that these hurdles can also be successfully overcome. In the following, butanediol is presented as an example of a commercial bio-based bulk chemical.
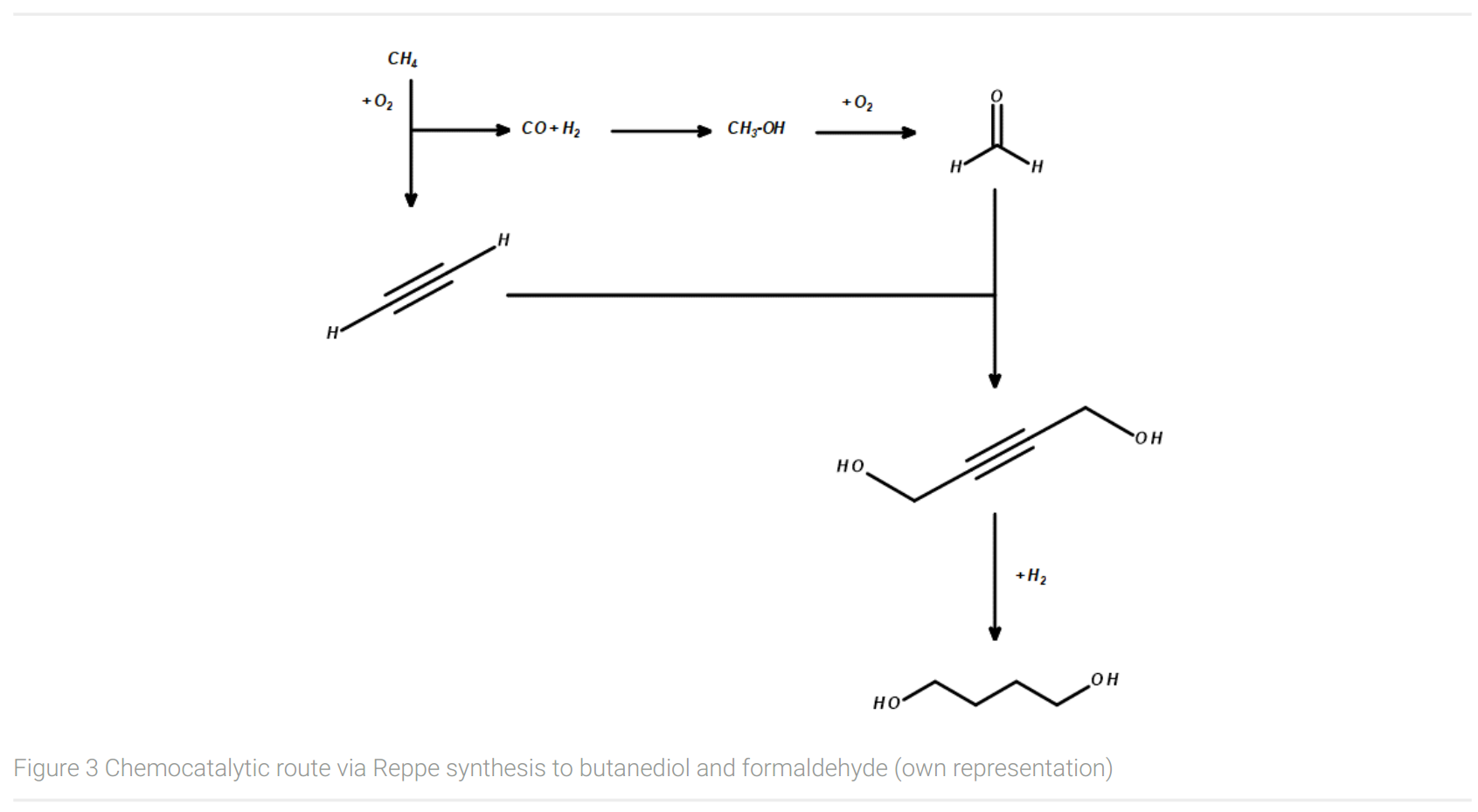
Figure 3 shows an important chemocatalytic route to butanediol and formaldehyde via Reppe synthesis (Arpe, 2007). Today, one of the industrial, large-scale synthesis route for BDO using fossil-based feedstocks begins with the conversion of methane from natural gas with oxygen to acetylene and syngas (CO and H2). In a second step, syngas reacts to methanol under catalytical conditions. Formaldehyde is being produced by the conversion of methanol with oxygen with a silver or Fe/Mo/O catalyst. In the following synthesis step, formaldehyde is being added to acetylene with the usage of a copper catalyst to yield in 1,4-butynediol. Finally, butynediole is hydrogenated with H2 to give 1,4-butanediol.
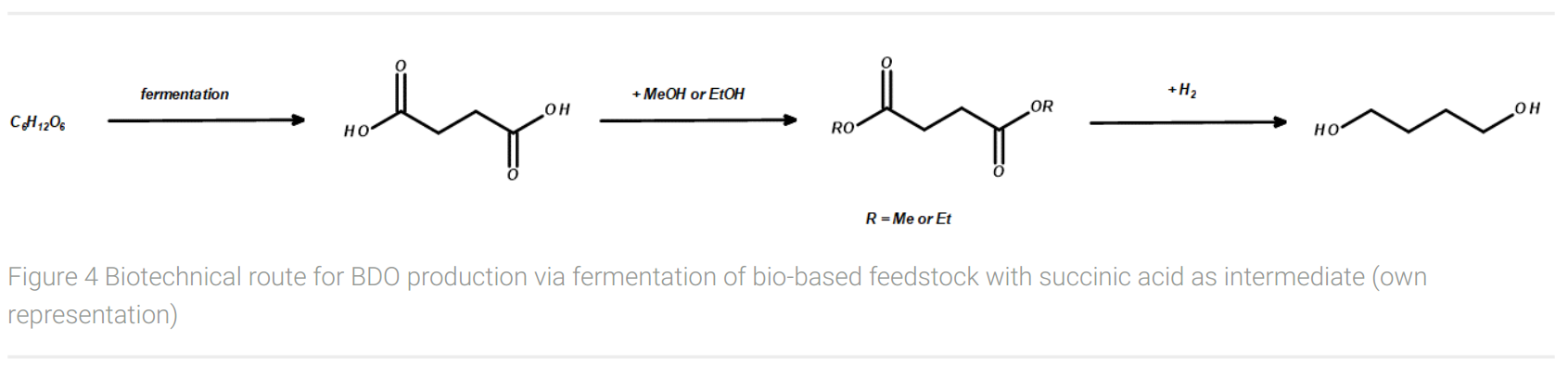
A biotechnological route for the production of BDO from a biobased feedstock at a scale of several kilotons was introduced through the collaboration of BASF and Purac (Figure 4). This fermentation process of renewable feedstocks such as glycerol, dextrose syrup, or maltose does not result in BDO, but in succinic acid giving a yield of minimum 1.0 g/g glycerol (Murzina and Ingram, 2017). With a theoretical yield of 1.28 g/g glycerol, the yield is about 80%. From succinic acid, however, two further synthesis steps are required to produce the desired 1,4-butanediol. Since direct reduction is not possible in a large-scale production process, the first step is esterification, e.g. with ethanol to form succinic acid ethyl ester. This ester is finally hydrogenated with H2 to give BDO and ethanol, the latter of which can be recycled for the esterification of succinic acid.
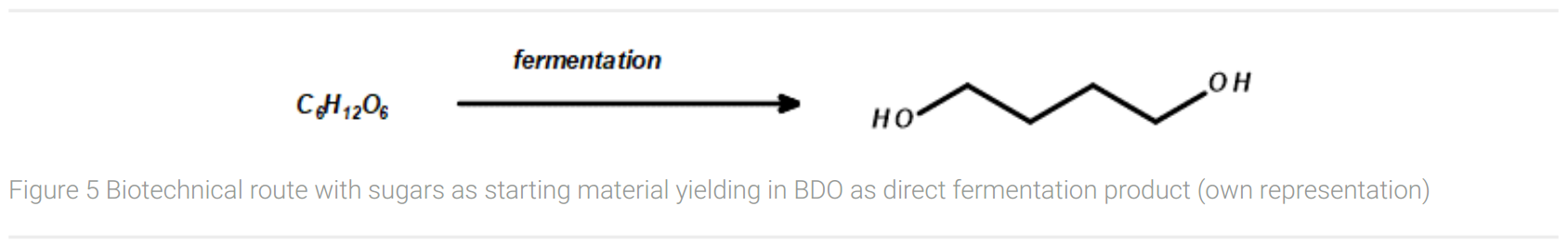
The large-scale biotechnological production route for BDO today is the Genomatica process. Together with Novamont, a 30 kt production plant for biofeedstock was started up in Italy in 2016. Starting from glucose or molasses, the fermentation process with the biotechnologically produced BDO pathway in E. coli delivers the desired 1,4-butanediol with a final concentration of up to 140 g/L (Figure 5). (Culler, 2016) However, exact yields are not published by Genomatica. Starting from glucose (six carbon atoms), two molecules of carbon dioxide are released per molecule of 1,4-butanediol (four carbon atoms).
3.3 Sustainable supply of bio-based bulk chemicals
BASF is one of the leading BDO producers with an annual capacity of 190,000 tons in Germany (PERP Report, 2004). Switching total BDO production to sugar-based fermentation would result in a sugar demand of 350,000 tons of sugar (roughly estimated at 53% yield) (Forte et al., 2016). Measured against German sugar production of around 4.3 million tons (2019/2020), the supply of only bio-BDO would therefore take up 8% of the harvest.
If butanediol, as just one example of a high-volume chemical, is already taking up a significant portion of the sugar crop, the question arises whether and how the necessary raw materials can be provided in a sustainable manner. After all, it has to be taken into account that the chemical industry, as a major buyer of raw materials, is entering a market to be supplied by the very same arable land area that today at 86% is used for the production of food and feed (Bioplastics study, 2017). Increasing production or expanding acreage is limited because natural resources are already stretched to the limit, and in some cases beyond, in terms of ecosystem services (Costanza, 2014) and planetary boundaries (Rockström, 2009; Steffen, 2015). Concerning greenhouse gas mitigation, it should be considered that agriculture is a significant emitter, and that intensification of farming inevitably will increase emissions. The future supply of biobased carbon sources to the chemical industry as a whole should increasingly be based on cascading and recycling according the principles of a circular economy (European Commission study, 2018). Alternatives in this regard are offered by non-food biomass, industrial residuals, and waste materials. Options will be presented by the example of biobased BDO in the following.
3.4 Sustainable options of biogenic carbon sources
3.4.1 Lignocellulosic sugar
One option is the utilization of lignocellulolic non-food biomass from either agricultural residues such as straw, corn cobs, or rice husks or woody residues from forestry or wood processing and green cuttings from municipal waste management. After separating lignin and the cellulose fraction (hemicellulose, cellulose) the C5– and C6-sugars, that build the cellulose fraction, are released (Kucharska et al., 2020). C5– and C6-sugars are to be transformed to bio-BDO or other chemicals in principle the same way as sugar from sugar crop. Concerning the lignin fraction methods to produce various chemicals (Yu and Kim, 2020) or construction materials (Wu et al., 2020) are under development.
3.4.2 Methane
In the medium term, another pathway to bio-based BDO from renewable feedstocks can be considered. Starting from biobased methane, which can be produced by anaerobic fermentation of organic waste, e.g. from agricultural residues, livestock farming, food waste etc., a wide variety of organic materials becomes available as raw material for further processing. The anaerobic fermentation to biogas (bio-methane) standardizes the various starting materials to a uniform carbon source, which is rich in energy, established as a starting material for chemical syntheses, easily transportable, and storable. In this respect, biogas can play a key role in closing the technical carbon cycle.
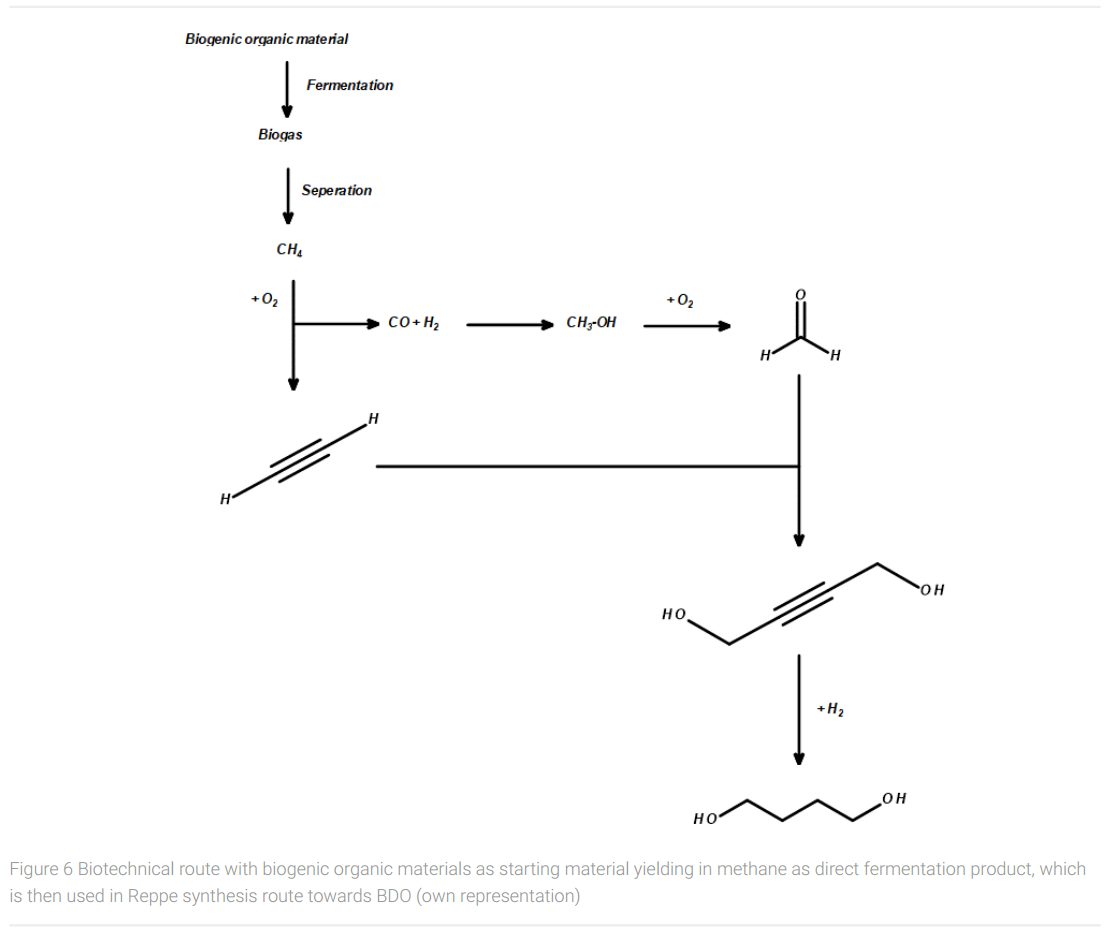
In the case of producing bio-BDO from methane, the classic chemical Reppe synthesis route via acetylene, syngas, methanol, formaldehyde, and 1,4-butynediole can be applied for the large-scale manufacturing of biobased BDO (Figure 6). The only raw material missing in this synthetic route is hydrogen for the reduction of the triple bond in 1,4-butynediol to yield in 1,4-butanediol. The hydrogen required for this can be produced by conventional water electrolysis using electricity if sufficient emission-free power is available. Alternatively, hydrogen could be obtained from biomass pyrolyzed and gasified in a gasifier reactor in a similar way as for coal, (Meramo-Hurtado et al., 2020) avoiding the use of conventional fossil raw materials. Also, biotechnological pathways to hydrogen using algae and bacteria are under development but still not ready for industrial practice (Gupta et al., 2013).
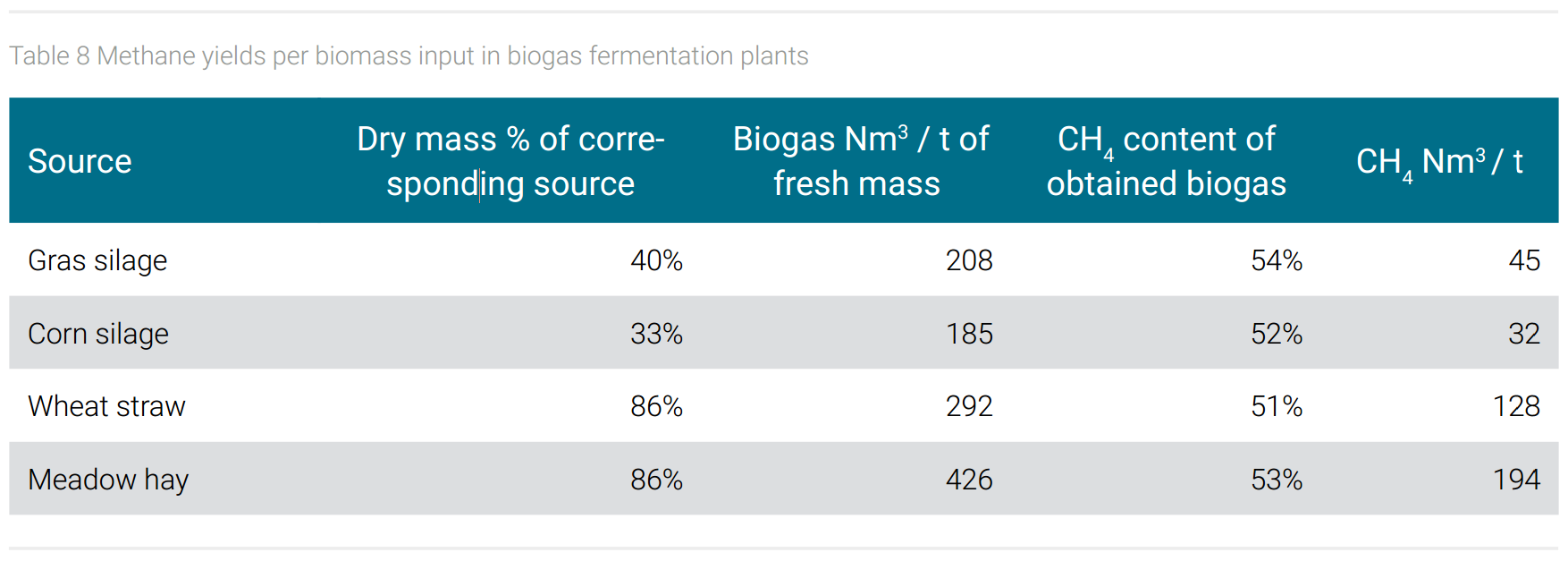
The demand of biogenic material to produce methane from biogas plants can only be estimated, due to the fact that different biogenic feedstocks give different biogas yields. The production of 190,000 tons of 1,4-butanediol requires 904 million Nm3 methane (with a given density of 0.7175 kg per 1 Nm3 methane). Estimating an average yield of 100 Nm3 of methane per ton of biogenic starting materials (estimating an average methane content of 52% in the obtained biogas and calculated as average mixture of raw materials shown in table 8 as dry mass input), one would need 4.7 million tons of biogenic feedstock for the production of 190,000 tons of BDO.
In the long term, basic chemical building blocks such as methane or methanol should be accessible in a CO2-neutral way by reducing carbon dioxide or even by capturing CO2 out of the atmosphere. The conversion of CO2 with water to methanol is already experimentally available in lab scale. This process consumes 5.85 MWh/t methanol (Pant et al., 2020), oxygen is derived hereby as a by-product. When the basic chemicals methane and methanol from carbon dioxide are available, the mature established, and optimized synthesis route can be used for large-scale production of BDO and formaldehyde in a sustainable manner.
3.4.3 CO2
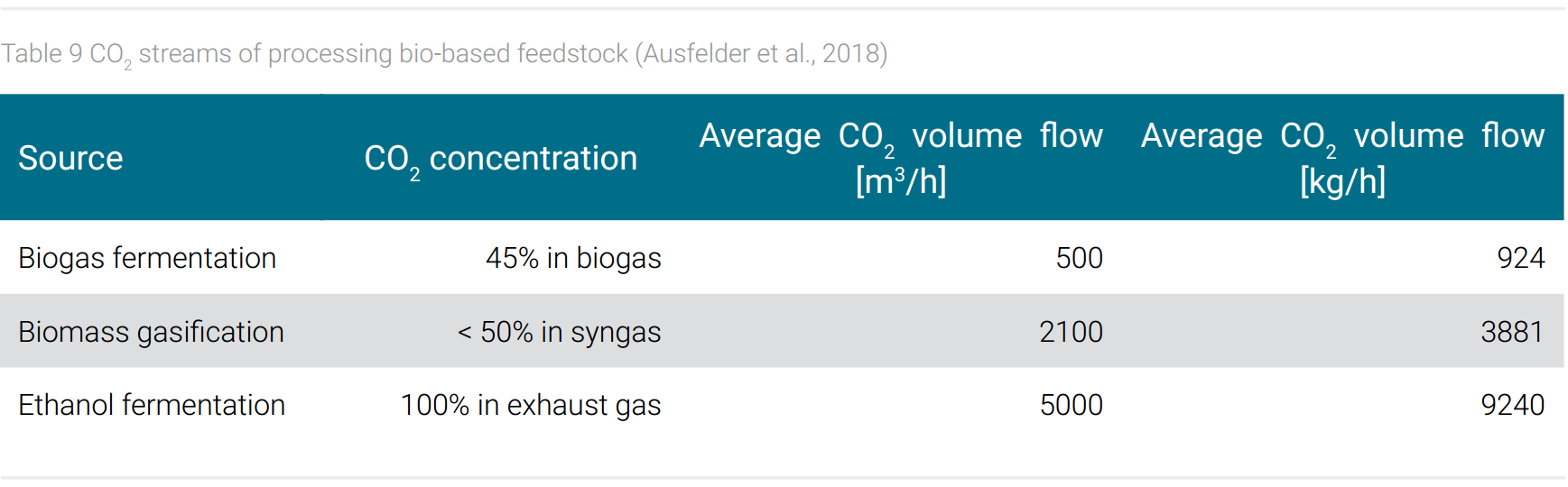
In addition to atmospheric CO2, emission streams from technical plants can also be directly biologically bound. Examples are the cultivation of algae to produce fatty acids as a source for biodiesel (Khan et al., 2017) or fine chemicals (Seungjib et al., 2017). These photosynthetic organisms use light as energy source. Also, some bacteria are able to metabolize CO2, for example into bioethanol, if hydrogen is provided as an energy source. One advantage of these processes is that CO2 emitted from one process can be fed directly into another technical unit, thus avoiding emission into the atmosphere. Examples for suitable CO2-emission streams are given in table 9 (Ausfelder et al., 2018). Another advantage is the robustness of these biological systems, which allow the use of polluted emission streams that would inactivate synthetic catalysts. Another option is technical photosynthesis, which converts CO2 into chemical products also by the use of microbes using electrical power (Haas et al., 2018).
Actual research efforts focus on the catalytic transformation of CO2 with hydrogen to formaldehyde (DECHEMA, 2019). In this approach, CO2 is catalytically reduced with hydrogen to give with two equivalents of methanol dimethoxymethane as an intermediate. The hydrolysis of dimethoxymethane yields in one part of formaldehyde and two parts of methanol, which is used for the first synthesis step in the first loop. The net reaction therefore is CO2 + H2 → HCHO + H2O. The described Ruthenium catalyzed reaction approach could utilize up to 1.46 tons of CO2 per ton of formaldehyde and is, therefore, suitable for the conversion of a greenhouse gas to a basic chemical.
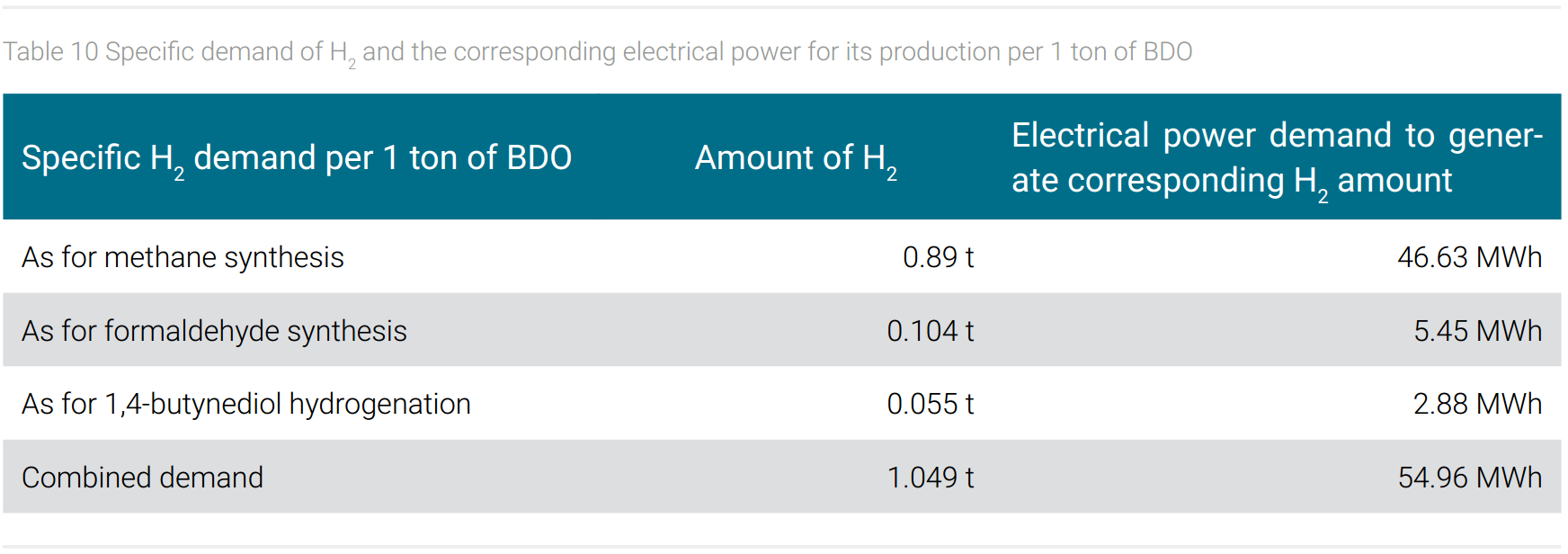
However, CO2 as a carbon source also has a price, because the provision of hydrogen is very energy-intensive. This will be explained using the example of butanediol and formaldehyde, since hydrogen is required for the synthesis of the precursor of formaldehyde (methanol from CO hydrogenation) for the final synthesis of 1,4-butanediol (hydrogenation of 1,4-butanediol) and for the hydrogenation of carbon dioxide to obtain methane as a C1 synthesis block. In table 10, the specific demand of hydrogen and the corresponding electrical energy for generating the amount of hydrogen per 1 ton of BDO is shown.
3.4.4 CO
Syngas, mainly consisting of CO, is another potential industrial carbon source. If produced by gasifying biogenic organic materials, it is a biogenic carbon source. Microbes like Clostridia, which have already been mentioned for metabolizing CO2, are able to use CO as well, even without offering hydrogen as energy source (Stoll et al., 2019; Abubackar et al., 2015).
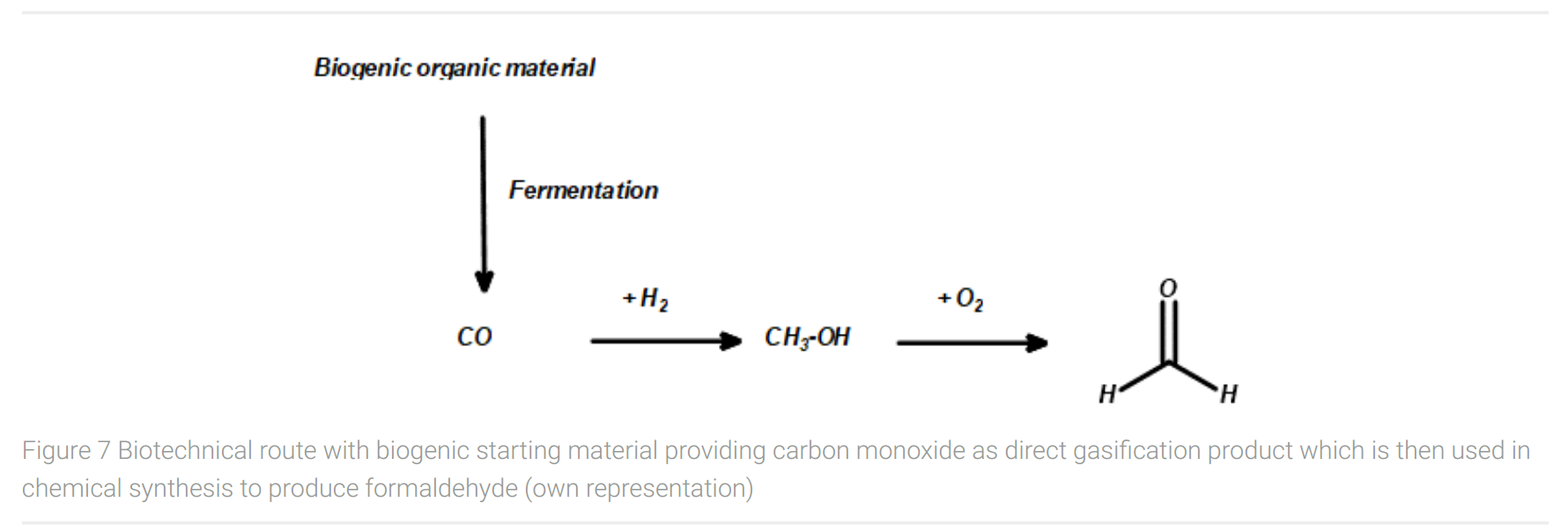
If this technology is available along with a scalable process, this would open the possibility for a combined biotechnological and chemical route to formaldehyde (Figure 7). This route starts with biogenic organic material as feedstock, which after gasification yields carbon monoxide. Carbon monoxide can be reduced to methanol with hydrogen. As mentioned before, the utilized hydrogen should be produced with power supply from renewable energy sources, e.g. in a water electrolysis process, to ensure the sustainable approach of the entire production process.
The synthetic pathway of carbon monoxide to BDO via Reppe synthesis would require the conversion of CO either to acetylene in a direct way or CO conversion to methane. While the first route is unknown yet, the latter process – conversion of carbon monoxide to methane – is subject of actual research (Mok, 2013). However, acetylene synthesis from methane with oxygen produces syngas (mixture of carbon monoxide and hydrogen) as the main product. The gained syngas could be used in the production of formaldehyde via methanol as an intermediate, as described above, to avoid any by-products and to establish a sustainable approach of the entire production process.
4 Conclusion and Outlook
There is widespread agreement in politics, industry, the scientific community, and society in general, that greenhouse gas emissions must be drastically reduced in the interests of climate protection. This includes the chemical industry, which has been subject to a tightening emissions regime under the European Emissions Trading Scheme since 2005. This applies above all to scope 1 and 2 emissions, which primarily concern emissions from generating energy. Therefore, it is obvious to switch energy production to emission-free energy sources and, as shown, many companies also follow this strategy.
There are several options for reducing scope 3 emissions which include among other things transport, and the use and disposal of consumer products. In the case of bulk chemicals, as discussed here, transport is a relevant source of emissions, because both, BDO and formaldehyde are intermediate products which have to be transported from plant to plant in the multi-stage processing chain to e.g. polymers or crop protecting agents. Transport emissions are reduced if the receiving chemical plant for further processing is located close to the production site, ideally even in the same chemical park, and the advantages of the Verbund model are used.
Emissions from waste management of consumer goods can be reduced by recycling if the polymers in these products can be separated from each other. This is a characteristic that must be taken into account as early as the product design stage.
Another option is to use biogenic raw materials for the carbon bound in the products. This is state of the art, particularly in the fine and specialty chemicals sector. However, significant reduction of the chemical sector’s scope 3 emissions can only be expected once the raw material base of large-volume basic chemicals and products such as the butanediol discussed in this article has also been converted to a more sustainable basis. If this option were to be comprehensively realized, it is clear that the chemical sector would evolve from an insignificant consumer of agricultural feedstock today to a very large one. This may lead to conflicts with the supply of food, land-use changes, and increased emissions of agricultural greenhouse gases, and thus could violate planetary boundaries and further damage ecosystem services that are already at risk. An exclusive focus on raw material change to primary biomass in the chemical industry therefore does not seem advisable.
The use of CO2 could have a relieving effect. However, the necessary technologies are still lacking. In addition, these processes will be energy-intensive and will depend on the availability of emission-free power. Concerning costs, biobased BDO and formaldehyde are still economically not competitive to their fossil-based equivalents due to comparatively high feedstock cost.
The competitive position of the established biobased processes, such as the Purac or Genomatica process, would improve if the ETS were to burden the scope 3 emissions of processes and products that are still fossil-based today.
Finally, it is to be discussed, which of the suggested options for a non-fossile feedstock supply for BDO and formaldehyde is to favour and to strike for in the long term. In the authors´ opinion, there is no single and simple answer for this. The favored synthesis route has to be chosen by the given regional and regulatory conditions. For example, in a regional setting that is able to provide non-food biomass in sufficient quantities for industrial purposes, in a sustainable manner and at competitive prices, the transformation of biomass to biogas, the separation of bio-methane and its further processing to BDO and formaldehyde would be the preferred option. However, the further processing of biogas respectively of the separated bio-methane to chemical products can be done efficiently in large scale chemical plants, utilizing the economy of scale and already installed equipment. Therefore, an infrastructure for biogas transportation to such central chemical plants has to be installed. Where available, using the existing grid for natural gas is an option.
In another scenario, where the biomass amount is limited, but electrical power from emission-free energy sources is sufficiently available at competitive prices, the synthesis routes from carbon monoxide or carbon dioxide feedstocks can be preferred.
All options for switching the feedstock base to biogenic carbon sources, i.e., primary biomass from agriculture and forestry, biogas from residues, CO2 from industrial emission streams, and CO from waste, probably cannot by themselves meet the high demand for bulk chemicals, but they can contribute, depending on local conditions. In this context, the coming transition phase from fossil to biogenic feedstocks is a significant challenge, because for a long time both carbon sources, fossil and bio-based, will be on the market in direct competition.
In summary, an emission-neutral chemical industry must avoid both energy- and product-related scope 1-3 emissions. Biogenic carbon sources can contribute together with technical carbon recycling. To ensure that the comparatively high feedstock, respectively, transformation costs of these carbon sources do not manifest themselves as a competitive drawback, and thus block the realization of these sustainability measures, the economic policy framework must be adapted to the requirements of a climate-friendly chemical industry. A globally coordinated system of greenhouse gas pricing scope 1-3 is a key element in this.
References
Abubackar, H. N., Veiga, M. C., Kennes, C. (2015) Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid, Bioresour. Technol., 186, pp. 122-127.
Arpe, H.-J. (2007), Industrielle Organische Chemie, Vol. 6, Wiley-VCH.
Ausfelder, F., Dura, H. E., Dechema study (2018): Optionen für ein nachhaltiges Energiesystem mit power-to-x Technologien, available at: https://dechema.de/dechema_ media/Downloads/Positionspapiere/2018_Power_to_X.pdf, accessed 16 March 2021.
Austin, K. G., Baker, J. S., Sohngen, B. L., Wade, C. M., Daigneault, A., Ohrel, S. B., Ragnauth, S., Bean A. (2020) The economic costs of planting, preserving, and managing the world’s forests to mitigate climate change, Nature Comm. 11, article number: 5946.
BASF (2019): Committed to your success, available at https://www.basf.com/global/en/products/chemicals.html, accessed 16 March 2021.
BASF (2020): Emissionsreduktion entlang der Wertschöpfungskette, available at https://www.basf.com/ global/de/who-we-are/sustainability/we-produce-safelyand-efficiently/energy-and-climate-protection/reducingemissions-along-the-value-chain.html, accessed 16 March 2021.
BASF (2021): Our climate protection goal, available at https://www.basf.com/us/en/who-we-are/sustainability/ we-produce-safely-and-efficiently/energy-and-climateprotection/climate-protection-goals.html, accessed 16 March 2021.
BASF (2021): Wo wir stehen: Die BASF-CO2 -Bilanz 2020, available at https://www.basf.com/global/de/who-we-are/ sustainability/we-produce-safely-and-efficiently/energyand-climate-protection/corporate-carbon-footprint.html, accessed 16 March 2021.
Bioplastics study (2017): Land use estimation for bioplastics in 2017 and 2022, available at https://www.europeanbioplastics.org/how-much-land-do-we-really-need-toproduce-bio-based-plastics/, accessed 16 March 2021.
Costanza, R. (2014) Changes in the global value of ecosystem services, Global Environmental, 26, pp. 152-158.
Culler, S. (2016) Che. Eng. Progress, 9, pp. 42-51.
DECHEMA (2019): CO2 Plus – Stoffliche Nutzung von CO2 zur Verbreiterung der Rohstoffbasis, available at https://co2- utilization.net/uploads/CO2plus_Abschlussergebnisse.pdf, accessed 16 May 2021.
DECHEMA (2019): Roadmap Chemie 2050, available at https://dechema.de/dechema_media/ Downloads/Positionspapiere/2019_Studie_Roadmap_ Chemie_2050-p-20005590.PDF, accessed 16 March 2021.
Deerberg, G., Hensmann, M., Sprecher, M., Hoppe, H., Hannes, J., Merretig-Bruns, U., Sayder, B., Schwarze-Benning, K., Körner, H.-J. (2020) Möglichkeiten der Integration in bestehende industrielle Anlagen mit relevanten C1 – Gasströmen, in: Kircher, M., Schwarz, T. (ed.) CO2 und CO – Nachhaltige Kohlenstoffquellen für die Kreislaufwirtschaft, Springer, pp. 169-218.
European Commission study (2018): Expert Group Report Review of the EU Bioeconomy Strategy and its Action Plan, available at https://gbs2018.com/fileadmin/gbs2018/ Downloads/Expert_group_report.PDF, accessed 16 March 2021. Evonik (2020): Evonik carbon footprint 2019, available at https://corporate.evonik.de/downloads/corporate%20 responsibility/evonik_carbon_footprint_de.pdf, accessed 16 March 2021.
Evonik (2020): Top 10 Nachhaltigkeitsziele, available at https://corporate.evonik.com/Downloads/Corporate%20Responsibility/Nachhaltigkeitszeile%20dt%20Überblick%20 groß.jpg, accessed 16 March 2021.
Evonik (2021): Leading beyond chemistry, available at https://corporate.evonik.de/en/company, accessed 16 March 2021.
FNR (2021): Basisdaten biobasierte Produkte 2021, available at https://fnr.de/fileadmin/allgemein/pdf/broschueren/ basisdaten_biobasierte_produkte_2021_web.pdf, accessed 16 March 2021.
Forte, A., Zucaro, A., Basosi, R., Fierro, A. (2016) Materials, 9, pp. 563-570.
Gupta, S. K., Kumari, S., Reddy, K., Bux, F. (2013): Trends in biohydrogen production: major challenges and state-of-theart developments, Environ. Techno., 34(13-16), pp. 1653- 1670.
Haas, T., Krause, R., Weber, R., Demler, M., Schmid, G. (2018) Technical photosynthesis involving CO2 electrolysis and fermentation, Nature Catalysis, 1, pp. 32-39.
Henkel (2020): Sustainability report 2019, available at https://www.henkel.com/resource/blob/1038544/ af1684b38200328128a80b5dd18e485e/data/2019- sustainability-report.pdf, accessed 16 March 2021.
Henkel (2021): Brands and Businesses, available at https:// www.henkel.com/brands-and-businesses, accessed 16 Mach 2021.
Henkel (2021): Climate protection strategy and targets, available at https://www.henkel.com/sustainability/ positions/climate-positive, accessed 16 March 2021. Khan, S., Siddique, R., Sajjad, W., Nabi, G., Hayat, K. M., Duan, P., Yao, L. (2017),
Biodiesel Production From Algae to Overcome the Energy Crisis, Hayati J. of Biosciences, 24, pp. 163-167.
Krämer, D., Bazzanella, A. (2017): Technologien für Nachhaltigkeit und Klimaschutz – Chemische Prozesse und stoffliche Nutzung von CO2 , Dechema, pp 12-31.
Kucharska, K., Słupek, E., Cieśliński, H. (2020): Advantageous conditions of saccharification of lignocellulosic biomass for biofuels generation via fermentation processes, Chem. Pap., 74, pp. 1199–1209.
McKinsey (2007): Kosten und Potenziale der Vermeidung von Treibhausgasemissionen in Deutschland, available at https://www.mckinsey.com/~/media/McKinsey/Business%20Functions/Sustainability/Our%20Insights/Costs% 20and%20potentials%20of%20greenhouse%20gas%20abatement%20in%20Germany/Read%20the%20Deep%20 Dives%20Industry.pdf, accessed 16 March 2021.
Meramo-Hurtado, S., Puello, P., Cabarcas, A. (2020): Process analysis of hydrogen production via biomass gasification under computer-aided safety and environmental assessments, ACS Omega, 5, 31, 19667–19681.
Mok, Y. S. (2013): Production of methane from carbon monoxide in a plasma-catalytic combined reactor system, Int. J. Sus. Dev. Plann., 8, pp. 186–196.
Murzina, A., Ingram, A. (2017): PERP Report 1,4-Butanediol/ Tetrahydrofuran.
Nijnik, M. (2010): Carbon capture and storage in forests, in: Hester, R.E., Harrison, R. M. (ed.) Issues, in: Environmental Science and Technology, Vol. 29, Royal Society of Chemistry, pp. 203-239.
Pant, D., Bulut, M., de Wever, H. (2020) , in: Kircher, M., Schwarz, T. (ed.) CO2 und CO – Nachhaltige Kohlenstoffquellen für die Kreislaufwirtschaft, Springer, pp. 316-319.
Parisi, C., Ronzon, T. (2016): A global view of bio-based industries: benchmarking and monitoring their economic importance and future developments.
PERP Report (2004): 1,4-Butanediol/THF, 02/03-7, pp. 155- 156.
PERP Report (2016): Formaldehyde and resins, p. 66.
Rockström, J. (2009): Planetary Boundaries: Exploring the Safe Operating Space for Humanity, Ecology and Society, 14(2), pp. 32.
Scarlat, N., Fahl, F., Dallemand, J. F. (2019): Status and Opportunities for Energy Recovery from Municipal Solid Waste in Europe, Waste Biomass-Valorization, 10, pp. 2425–2444.
Seungjib, J., Byeongryool, J., Yong, K. C. (2017): Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals.
Steffen, W. (2015): Planetary boundaries: Guiding human development on a changing planet, Science, 347 (6223), p. 1259855.
Stoll, K., Boukis, N., Sauer, J. (2019): Syngas Fermentation to Alcohols: Reactor Technology and Application Perspective.
Tcvetkov, P., Cherepovitsyn, A., Fedoseev, S. (2019): Public perception of carbon capture and storage: A state-of-the-art overview, Heliyon, 5.
Van den Oever, M., Molenveld, K., van der Zee, M., Wageningen, H. B. (2017): Bio-based and biodegradable plastics: facts and figures, Food & Biobased Research, 1722, pp. 1-65.
VCI (2021): Rohstoffbasis der organischen Chemie in Deutschland, Anteile in Prozent, 2018. available at https:// www.vci.de/themen/rohstoffe/vci-statistik-energieklimaschutz-rohstoffe-in-der-chemie.jsp, accessed 16 March 2021.
World Resources Institute (2021): Corporate standard, available at http://www.ghgprotocol.org./standards/ corporate-standard, accessed 16 March 2021.
Wu, J., Liu, Q., Wang, C., Wu, W., Han, W. (2020): Investigation of lignin as an alternative extender of bitumen for asphalt pavements, J. Clean. Prod., 283, 124663.
Yu, O., Kim, K.H., (2020): Lignin to Materials: A focused review on recent novel lignin, Applications, Appl Sci., 10, p. 4626.